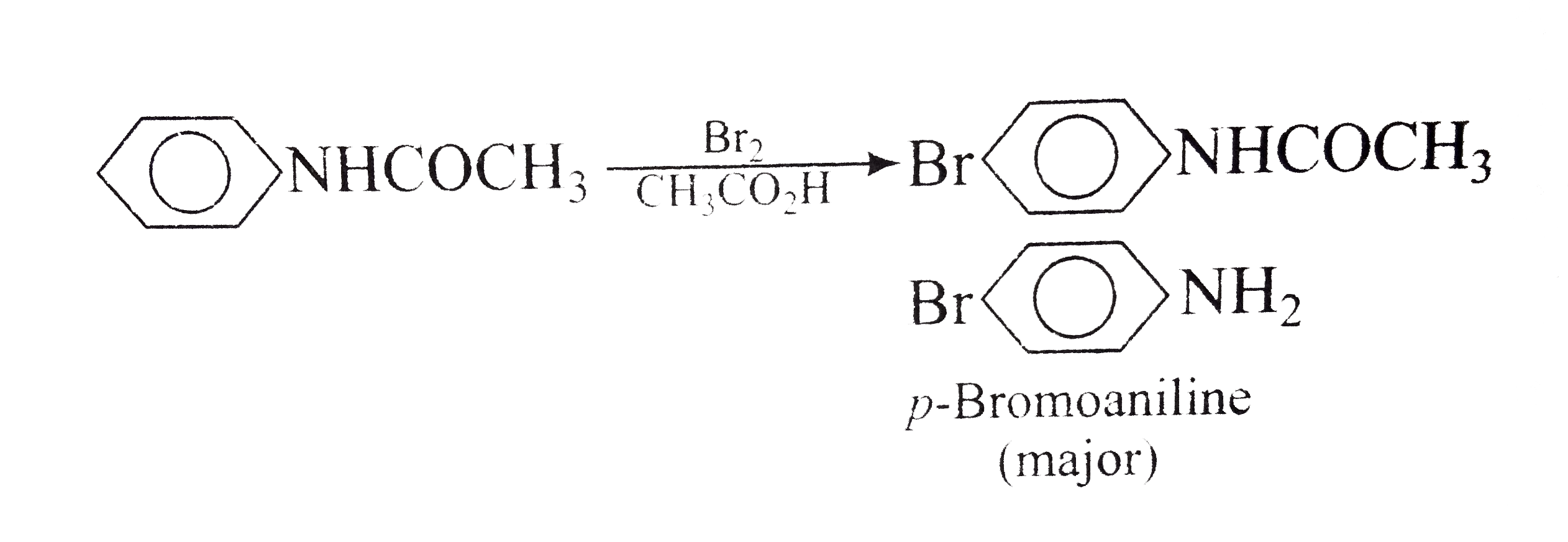ch3cooh + naoh equation
|
Agdal faculte des sciences rabat filière smpc s2 chimie générale ii
- Dosage d'un acide faible CH3COOH par une base forte NaOH CH3COOH (Na Va) avec Na = pCa p = 1 NaOH (Nb Vb) avec Nb = pCb |
|
CH3COOH/CH3COO
◇ le tirage de CH3COOH (01 mol L-¹) par NaOH ( 01 mol · L-¹ · ◇ le tirage d'HC1 (01 mol L-2) par NaOH ( 01 mol L-1); • • ◇ le tirage de CH3COOH (10- |
|
Chapitre 1 Acides et bases
CH3COOH = CH3COO– + H+ couple H3O+ /H2O : H+ + H2O = H3O+ CH3COOH + H2O ⇌ CH3COO– + H3O+ Le pKa du couple CH3COOH / CH3COO– vaut 48 à 25◦C 1 3 3 |
|
Corrigé
Comme la solution est obtenue après réaction entre NaOH et CH3COOH il faut que (1) Equation de la réaction ou équation du dosage: KOH + HClO4 K+ + ClO4 |
|
Cours-titrage-acide-base-2ème-annnée-pdf
Le pH est déterminé par la position de l'équilibre de dissociation de l'acide acétique [CH3COO-] pH = pKa + log [CH3COOH] Après addition de 50ml de NaOH la |
|
LES REACTIONS ACIDE-BASE 1 Le pH 11 Définition Le
La demi-équation acido-basique associée est : CH3COOH = CH3COO− + H+ Page 3 NaOH + H2O → Na+ + OH- 4 2 Acides faibles et bases faibles Les acides |
|
Les réactions acido-basiques
CH3COOH / CH3COO- C6H5COO- / C6H5COOH C6H5COOH / CH3COO- HCO3 -/ CO2 4 - La demi-équation acido-basique Généralisation sur un couple AH/A- : Le signe |
|
Titrations (CHEM 160)
CH3COOH+NaOH →CH3COO- + H2O Interaction of the string base with the weak acid species forces the reaction Because of this we |
Quel est le pKa du couple CH3COOH ch3cook ?
Le pKa du couple CH3COOH / CH3COO– vaut 4,8 à 25◦C.
Est-ce que CH3COO est une base forte ?
Acide faible et base faible
Parmi les solutions d'acide faible et de base faible couramment utilisées, on peut citer les deux suivantes : La solution d'acide éthanoïque (aussi appelée acide « acétique ») de formule (\\ce{H3O+_{(aq)}} + \\ce{CH3COO^{-}_{(aq)}}) est une solution d'acide faible.Pourquoi CH3COOH est un acide faible ?
Son acidité caractérisée en solution aqueuse par un pKa = 4,76 vient de sa capacité à perdre temporairement le proton de sa fonction carboxylique, le transformant ainsi en ion acétate CH3COO−.
C'est un acide faible.- Un couple acide‑base s'écrit de la façon suivante : ACIDE / BASE avec l'acide toujours à gauche et la base toujours à droite.
L'acide éthanoïque CH3COOH peut céder un proton H+ et se transformer en CH3COO–, l'ion éthanoate, qui est sa base conjuguée.
Le couple s'écrit CH3COOH/CH3COO–.
|
EXPERIMENT A7:VINEGAR TITRATION Learning Outcomes
concentration of sodium hydroxide. The chemical reaction between acetic acid and sodium hydroxide is given below: CH3COOH(aq) + NaOH(aq) ? CH3COONa(aq) + |
|
Lecture 15
Titrations are based on the acid/base neutralization reaction. CH3COOH (aq) + NaOH (aq). CH3COONa (aq) + H2O (l). CH3COOH (aq) + OH-. (aq). CH3COO-. |
|
Acid-Base Chemistry Brønsted-Lowry Acids & Bases
CH3COOH(aq) + H2O(l) ? CH3COO-(aq) + H3O+(aq) acid base conjugate Step 1: since NaOH is a strong base ... exchange reaction |
|
E17 Acid Ionisation Constant of Acetic Acid from Titration Curve
It is called the half-equivalence point. The pH at this point should equal the pKa value for acetic acid. A plot of pH against the amount of added NaOH is |
|
Sample Exercise 17.1 Calculating the pH When a Common Ion is
Solve: Stoichiometry Calculation: The OH– provided by NaOH reacts with CH3COOH the weak acid component of the buffer. Prior to this neutralization reaction |
|
CHM 2046 Answer Key – Practice Quiz 2 Answer all questions. Be
base-ionization equation for CH3COO- in water. CH3COO- (aq) + H2O (l) ? CH3COOH (aq) (b) (13 points) What is the final pH after 10.0 mL of 0.200 M NaOH. |
|
CHEM1102 Answers to Problem Sheet 8 1. (a) The titration is a 1:1
As acetic acid is a weak acid [H3O+] must be calculated: CH3COOH (iv) The addition of 50.0 mL of 0.100 M NaOH corresponds to the equivalence. |
|
Buffer Solutions Buffer Solutions
acetic acid (remember that acids react with bases). CH3COOH(aq) + H2O ? CH3COO-(aq) + H3O+(aq) ? Henderson-Hasselbach Equation ... mL NaOH added. |
|
Lab 25. Acid-Base Titration and Neutralization Reactions: What Is
For example when sodium hydroxide is added to acetic acid |
|
The great chem-mystery
So if we know how much NaOH we have added (the burette readings tell us) then we can calculate how much acetic acid was in the flask. The equation for the |
|
The Common-Ion Effect - College of Science
1 8 x 10-5 = [CH3COO-][H3O+]/[CH3COOH] M = amount of acetic acid that dissociates then X M of H3O+ and CH3COO- ions are formed 1 8 x 10-5 = (X + 2 5)(X)/(0 5 - X) Can we use the shortcut to the quadratic? 0 5M acetic acid / 1 8 x 10-5 = 27777 (which is greater than 100) 1 8 x 10-5 = (X + 2 5)(X)/(0 5 - X) ? (2 5)(X)/(0 5) 9 0 x 10-6 = 2 5X |
How do you balance the equation CH3COOH + NaOH?
Balance the equation CH3COOH + NaOH = CH3COONa + H2O using the algebraic method. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients.
What is the chemical formula for the reaction between CH3COOH and NaOH?
CH3COOH + NaOH = NaCH3COO + H2O might be a redox reaction. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
What is the product of CH3COOH + NaOH?
The mixture of acetic acid (CH3COOH) and sodium hydroxide (NaOH) works as a solution of weak acid and strong base and the products of this reaction are sodium acetate (CH3COONa) and water (H2O). This is an example of acid neutralization reaction with weak acid and strong base.
What happens when CH3COOH and NaOH are mixed together?
The mixture of acetic acid (CH3COOH) and sodium hydroxide (NaOH) works as a solution of weak acid and strong base and the products of this reaction are sodium acetate (CH3COONa) and water (H2O). This is an example of acid neutralization reaction with weak acid and strong base. Let’s focus on the following topics related to the above subjects.
| CH3COOH + H2O ' CH3COO- + H3O+ - University of Illinois |
| Acetic Acid Content of Vinegar: An Acid-Base Titration |
| CH3COOH + NaOH = CH3COONa + H2O - Chemical Equation Balancer |
| The Common-Ion Effect - College of Science |
| Chapter 3 Chemical Reactions - Texas A&M University |
| Searches related to ch3cooh + naoh equation filetype:pdf |
How do you balance the equation CH3COOH + NaOH?
- Balance the equation CH3COOH + NaOH = CH3COONa + H2O using the algebraic method.
. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients.
What is the chemical formula for the reaction between CH3COOH and NaOH?
- CH3COOH + NaOH = NaCH3COO + H2O might be a redox reaction.
. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
. To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button.
. The balanced equation will appear above.
What is the product of CH3COOH + NaOH?
- The mixture of acetic acid (CH3COOH) and sodium hydroxide (NaOH) works as a solution of weak acid and strong base and the products of this reaction are sodium acetate (CH3COONa) and water (H2O).
. This is an example of acid neutralization reaction with weak acid and strong base.
What happens when CH3COOH and NaOH are mixed together?
- The mixture of acetic acid (CH3COOH) and sodium hydroxide (NaOH) works as a solution of weak acid and strong base and the products of this reaction are sodium acetate (CH3COONa) and water (H2O).
. This is an example of acid neutralization reaction with weak acid and strong base.
. Let’s focus on the following topics related to the above subjects.


















![92849518 Acetic Acid in Vinegar - [PDF Document] 92849518 Acetic Acid in Vinegar - [PDF Document]](https://0.academia-photos.com/attachment_thumbnails/54424227/mini_magick20190117-16721-bb2paz.png?1547720961)