base hydrolysis of esters conditions
Hydrolysis is a most important reaction of esters.
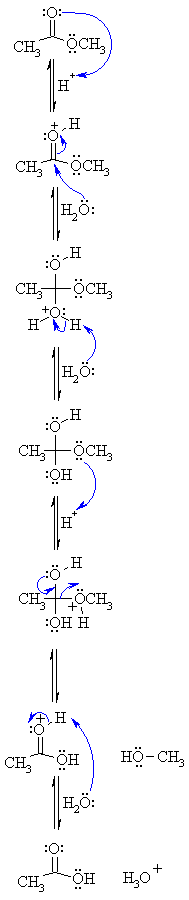
Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol.
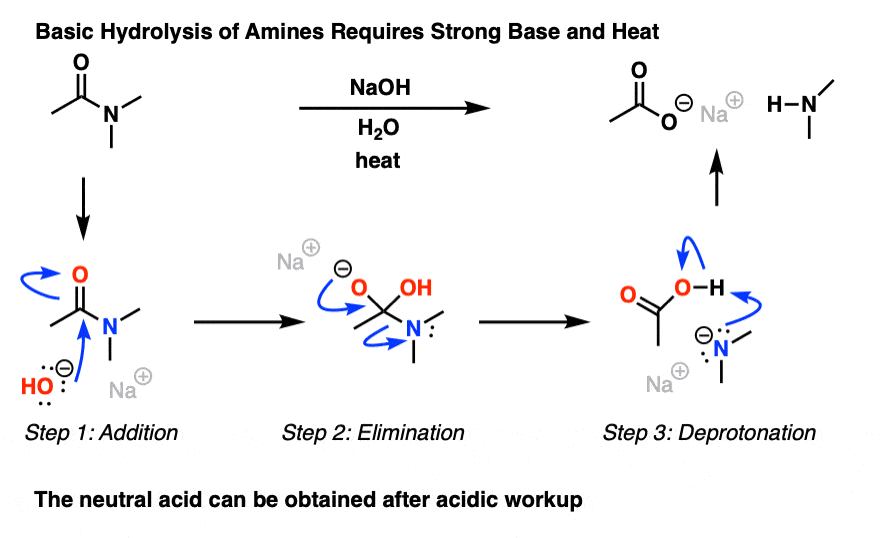
Basic hydrolysis of an ester gives a carboxylate salt and an alcohol.31 jan. 2022
What are the basic conditions for ester formation?
Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst.
The catalyst is usually concentrated sulphuric acid.
Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones where the carboxylic acid contains a benzene ring).
What kind of conditions can produce hydrolysis of an ester?
The hydrolysis of ester takes place in an acidic or basic medium.
In an acidic medium, ester interacts with the water molecule to give a carboxylic acid and alcohol.
In contrast, ester interacts with the water molecule to give a carboxylate salt and alcohol in a basic medium.
What are the factors affecting the hydrolysis of esters?
However, the rate of ester hydrolysis can be substantially increased by carrying out the reaction under acidic or basic conditions.
Since water is such a poor nucleophile, one method for increasing the rate of nucleophilic addition with an ester is to increase the electrophilicity of the ester.
|
A Mild and Selective Method for the Hydrolysis of Esters with
26 janv. 2005 elimination reactions induced by the often basic conditions employed. ... the hydrolysis of esters under extremely mild conditions that. |
|
Solvent Effects and Ester Interchange in Basic Hydrolysis of Esters
hydrolysis of the ester.1. To investigate separately with the saponification of esters in dioxane-water ... time i.e. |
|
Of the Base Hydrolysis of Various Amino Acid Esters Coordinated to
Reaction solutions under the following conditions of concentra- tion |
|
Mild alkaline hydrolysis of hindered esters in non-aqueous solution
Keywords: t-Butyl esters; non-aqueous conditions; saponification hindered esters; In a previous work |
|
Critical review of hydrolysis of organic compounds in water under
15 oct. 2009 Key words: Acid; base; environmental conditions; freshwater systems; hydrolysis; organic compounds; rate con- stants. Contents. |
|
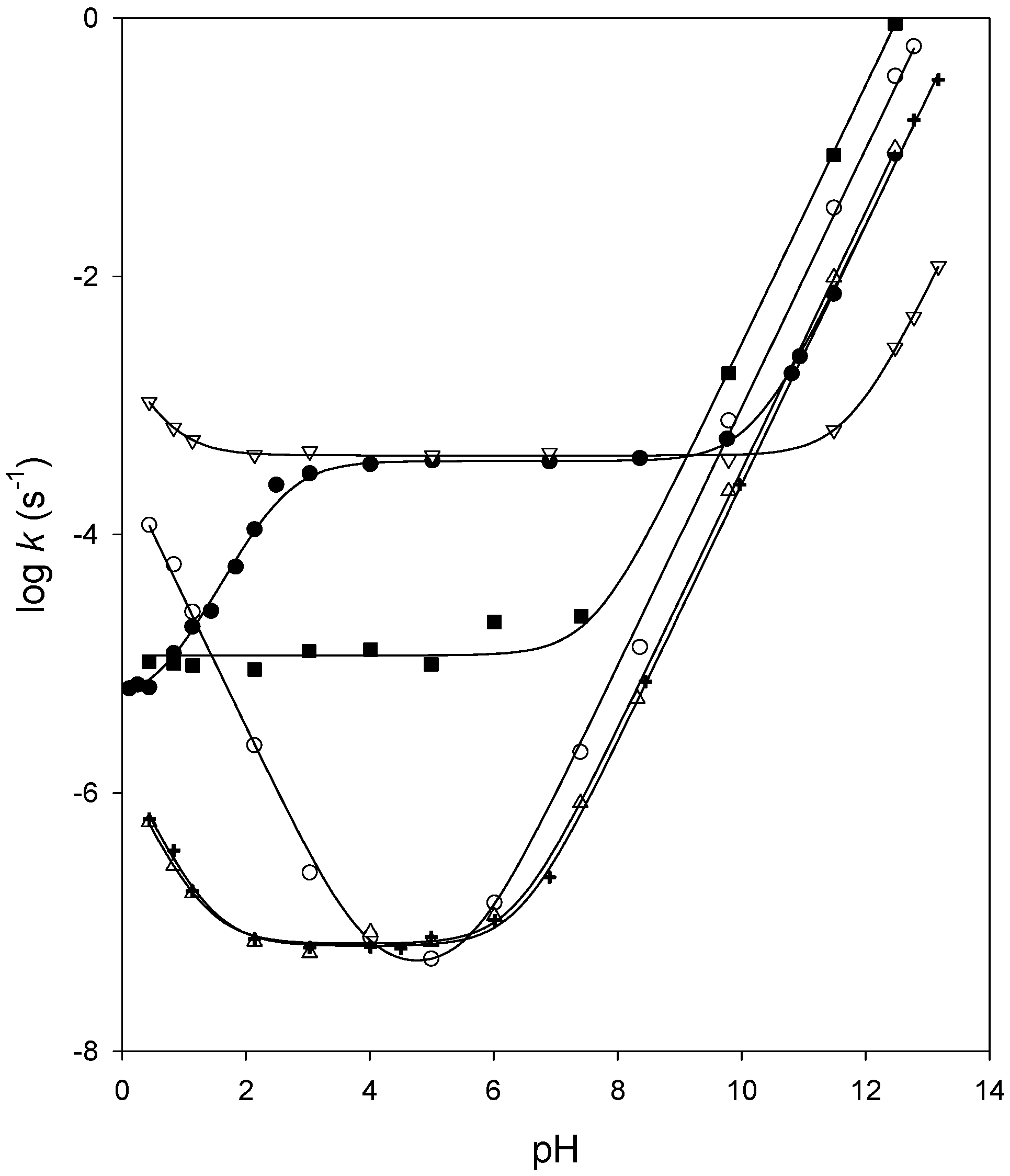
General Basic Catalysis of Ester Hydrolysis and Its Relationship to
The hydrolysis of ^-nitrophenyl acetate meets the requirements of general basic catalysis since the rate is pro- portional to the summation of catalytic |
|
Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green
27 avr. 2022 However standard cleavage of methyl esters requires either highly basic or acidic conditions |
|
Lecture 6: Hydrolysis Reactions of Esters and Amides - Objectives
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions;. • account for the irreversibility of the hydrolysis reaction under basic |
|
Efficiency of lithium cations in hydrolysis reactions of esters in
31 mars 2021 was an effective base for the hydrolysis of esters but did not explain the ... our reaction conditions despite THF generally showing ... |
|
Steric Effects. II. Base-Catalyzed Ester Hydrolysis - Marvin Charton
base-catalyzed hydrolysis of the esters. This assumption is basic to the Taft separation of electrical and steric effects. |
|
Lecture 6: Hydrolysis Reactions of Esters and Amides
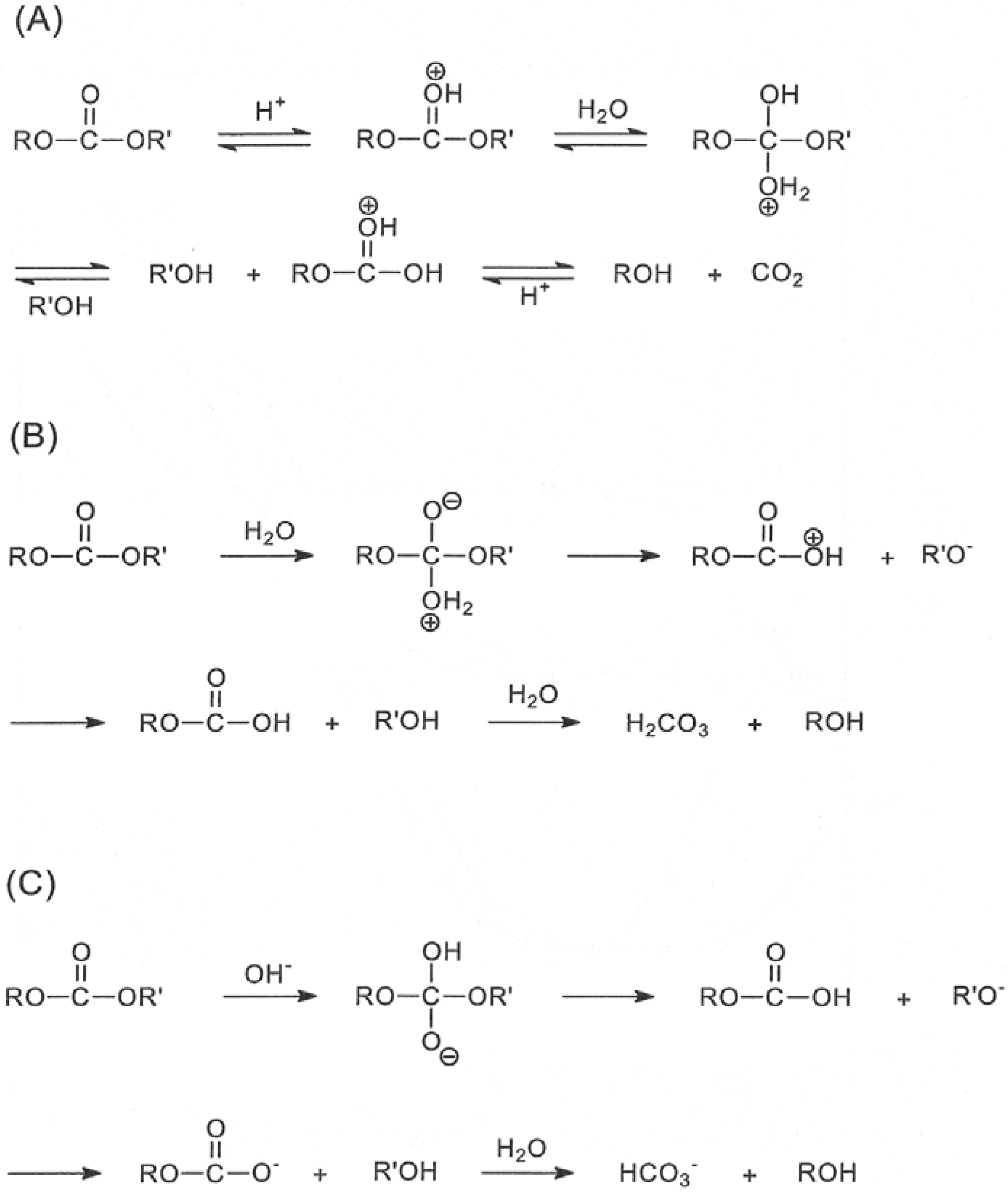
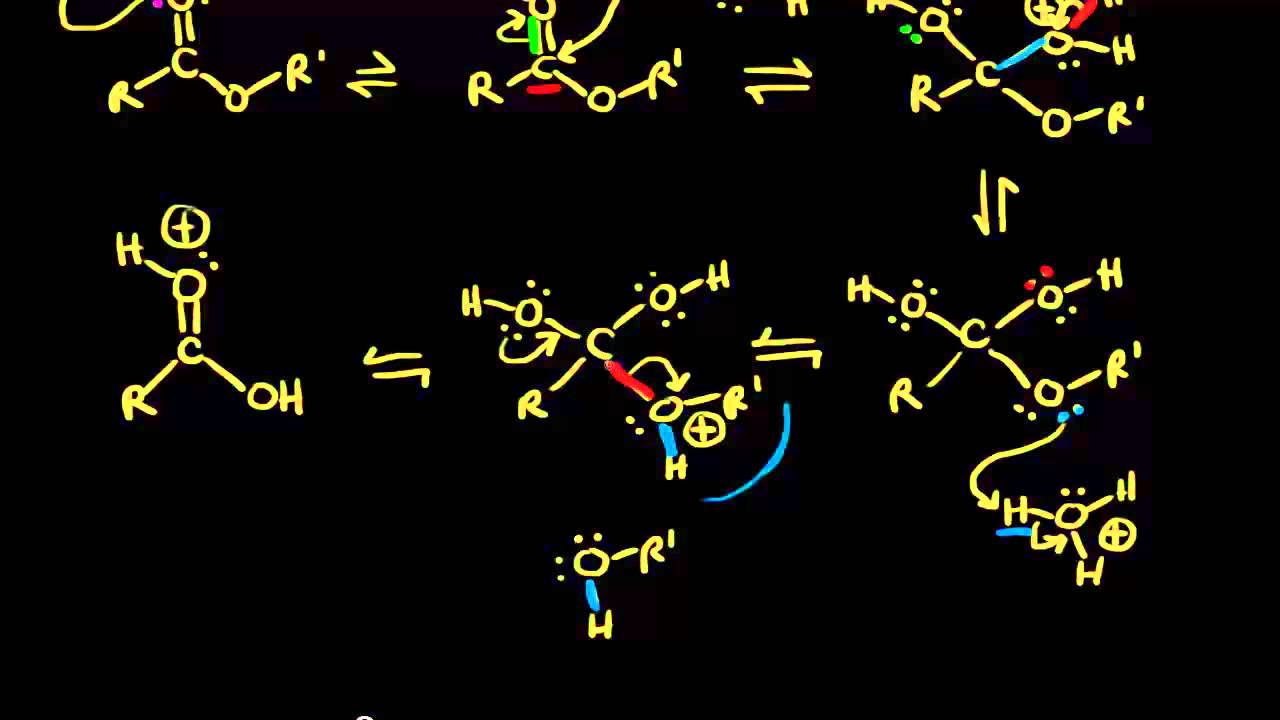
Simple alkyl esters, such as methyl and ethyl esters, are hydrolysed under acidic conditions in a reaction mechanism that is described as an AAC2 reaction: the reaction is acid-catalysed, the acyl−oxygen bond is cleaved and there are two molecules involved in the rate-determining step, i e it is a bimolecular process |
|
HYDROLYSIS
carbon centre, such as with carboxylic acid derivatives including esters, anhydrides, amides, mechanisms account for neutral, acid and base hydrolysis general, hydrolysis products predominant under neutral conditions, whereas |
|
Experiment C: Hydrolysis of a Carboxylic Acid Ester:
The hydrolysis of a carboxylic acid ester may proceed by a number of different mechanisms, depending on the substrate structure, the pH and the presence of catalyzing species (3-6) Under neutral conditions, the reaction generally proceeds via addition to the carbonyl carbon to produce a tetrahedral intermediate |
|
Mechanisms of Lactone Hydrolysis in Acidic Conditions
3 jui 2013 · 1 INTRODUCTION As is also the case with neutral and base-catalyzed mechanisms, the acid-catalyzed hydrolysis of esters has seldom been |
|
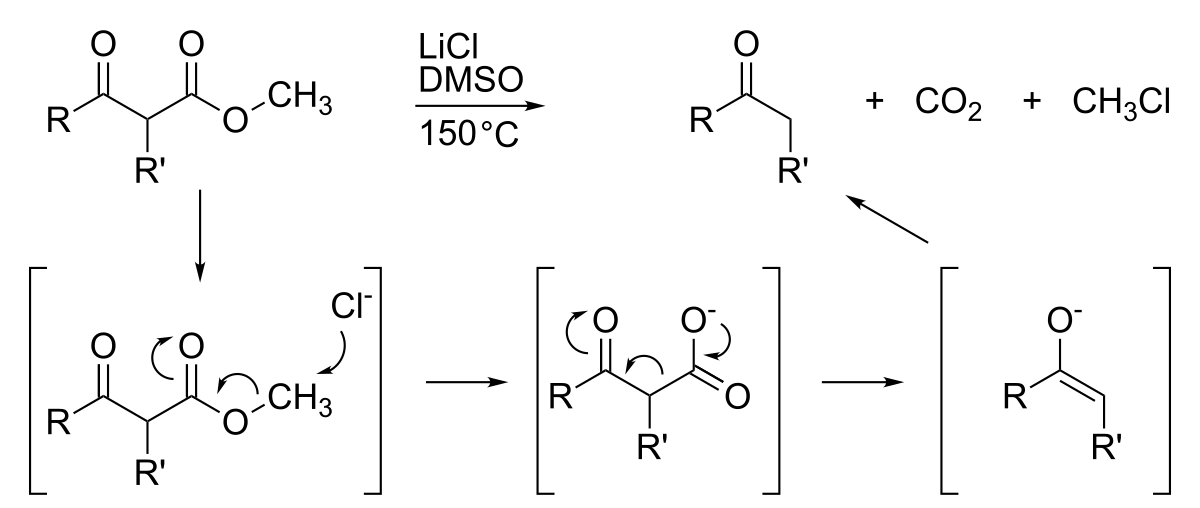
[2002] Intramolecular General Base Catalyzed Ester Hydrolysispdf
Significant buffer catalysis was not observed under conditions of the experiments (0 02 M buffer) The reactions were followed to completion, and infinity points |












![acid base catalysed Ester hydrolysis - [PDF Document] acid base catalysed Ester hydrolysis - [PDF Document]](https://0.academia-photos.com/attachment_thumbnails/46422824/mini_magick20190209-11202-1cnp8ew.png?1549775948)

![acid base catalysed Ester hydrolysis - [PDF Document] acid base catalysed Ester hydrolysis - [PDF Document]](https://d3i71xaburhd42.cloudfront.net/8649af16ade686f686e485c66b0dfb61aa569fb1/2-Figure2-1.png)
![PDF] Intramolecular general base catalyzed ester hydrolysis The PDF] Intramolecular general base catalyzed ester hydrolysis The](https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41467-019-09445-x/MediaObjects/41467_2019_9445_Fig1_HTML.png)



![PDF] Intramolecular general base catalyzed ester hydrolysis The PDF] Intramolecular general base catalyzed ester hydrolysis The](https://www.frontiersin.org/files/MyHome%20Article%20Library/427648/427648_Thumb_400.jpg)



