base hydrolysis of nitriles mechanism
What is the base catalyzed hydrolysis of nitriles?
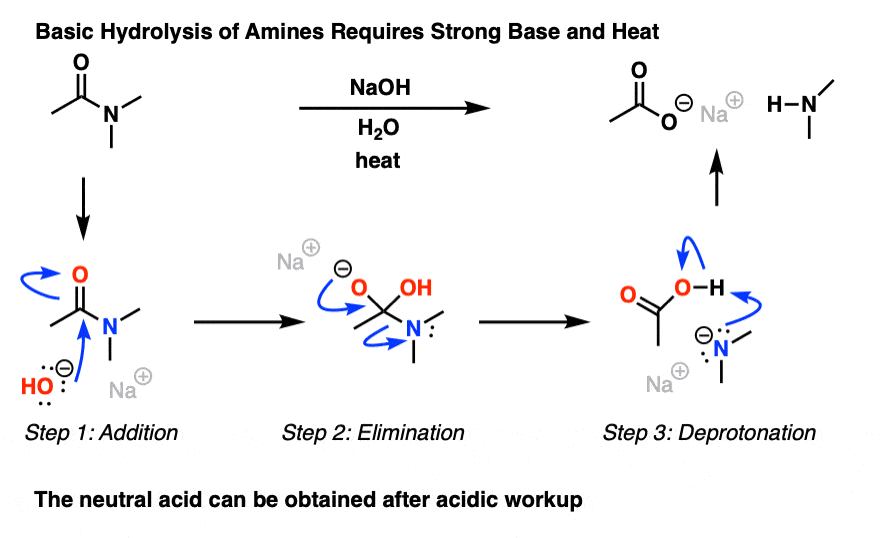
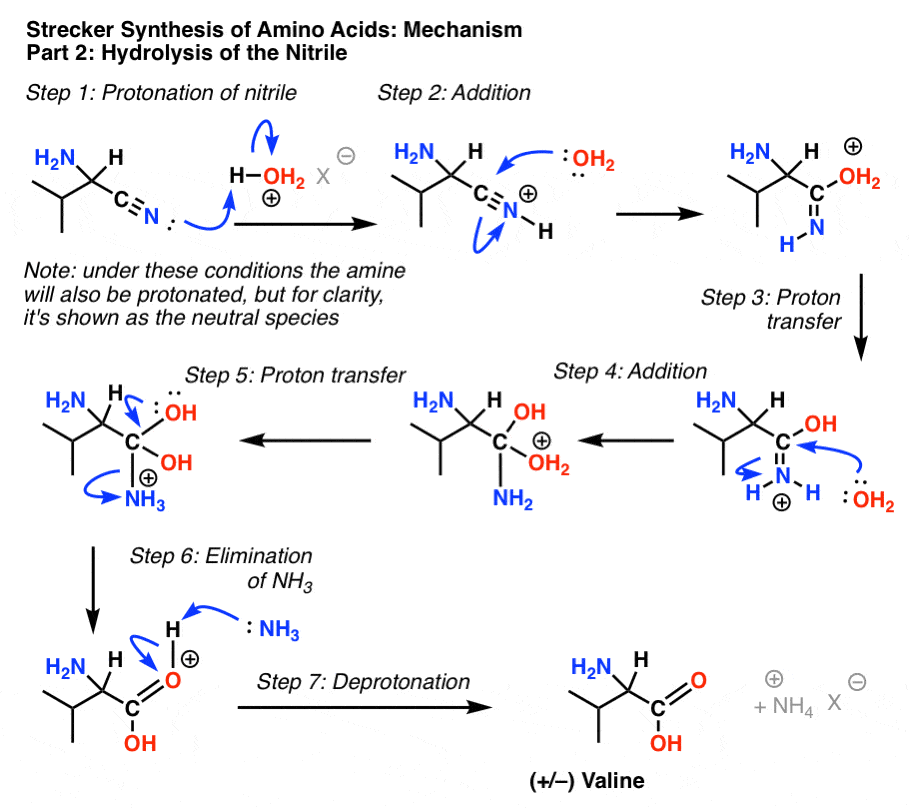
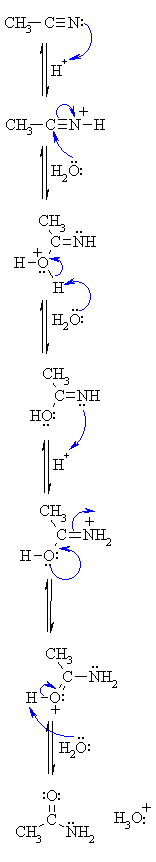
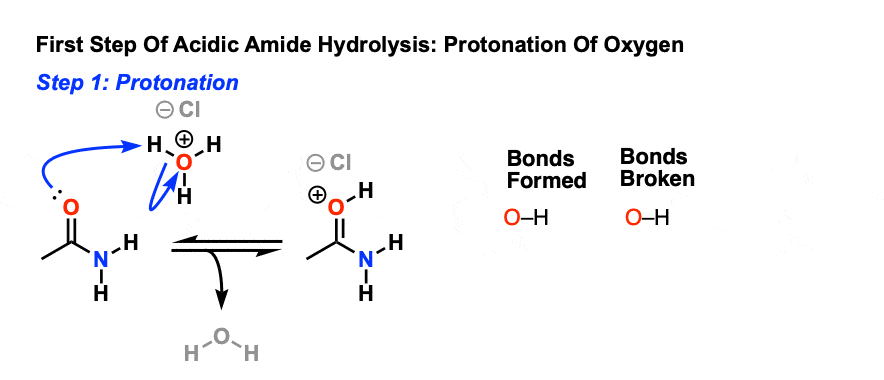
Nitriles can be hydrolyzed to carboxylic acids in acidic aqueous solutions, and to carboxylate salts with base-catalyzed hydrolysis: In both cases, the transformation consists of two main parts; conversion of the nitrile to an amide and hydrolysis of the amide to the corresponding carboxylic acid.
What is the mechanism of basic hydrolysis of nitriles?
Nitriles get hydrolysed in two steps; amides are formed first.
While in the second step, an ammonium salt of a carboxylic acid is formed.
For example, Ethanenitrile on getting hydrolysed gives ethanamide in the first step while ammonium ethanoate in the second step.The mechanism begins with the nucleophilic Grignard reagent reacting with the electrophilic carbon of the nitrile to form a salt of the imine anion.
The imine salt is protonated to form an imine which is subsequently protonated to form a positively charged iminium ion.2 fév. 2024
|
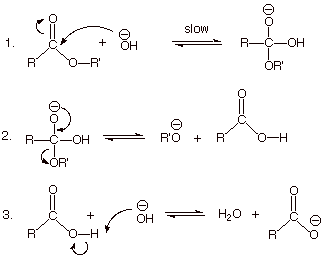
21.7 HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES
term saponification can be used to refer to the hydrolysis in base of any carboxylic acid derivative. The mechanism of ester saponification involves the |
|
20_18_20.html.ppt [Read-Only]
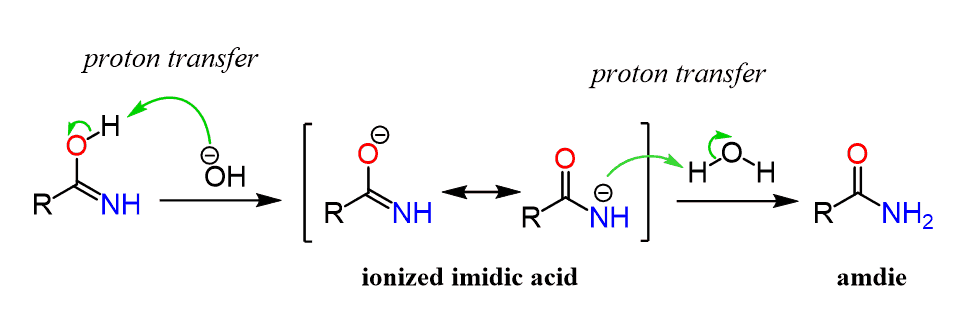
Hydrolysis of nitriles resembles the hydrolysis In basic solution the carboxylic acid product ... The mechanism of amide formation is analogous. |
|
Chem 360 Jasperse Ch. 20 21 Notes + Answers. Carboxylic Acids
Access: Alkyl or Aryl Acids. • Alkyl group can be 1º 2º |
|
Untitled
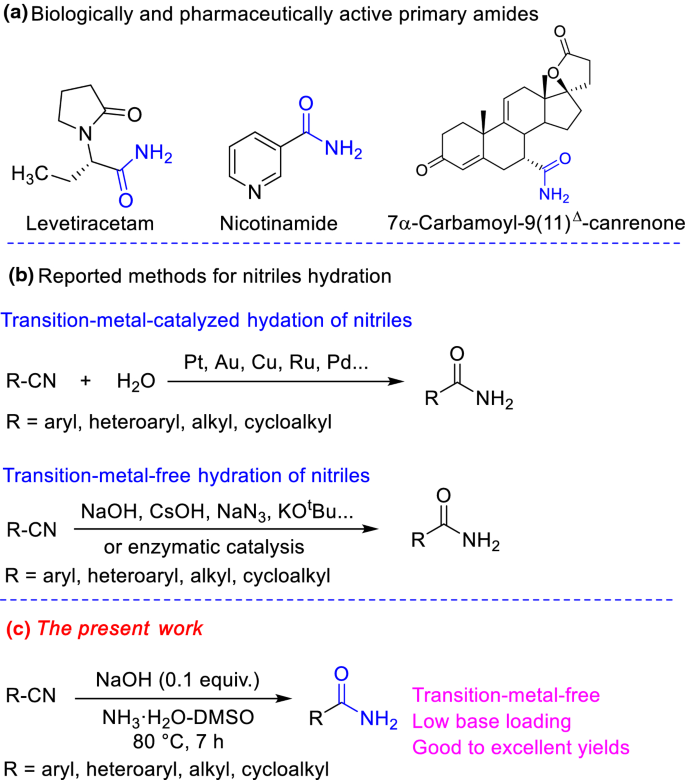
Mechanism of the Reaction of Nitriles with Alkaline Hydrogen Peroxide. and no peroxy acid a hydrolyzed product of 1 |
|
Reactivity of coordinated nitriles
Abstract: The base hydrolysis of coordinated acrylonitrile in 180 tracer studies indicate the mechanism of hydrolsis by carbonate ion to involve direct ... |
|
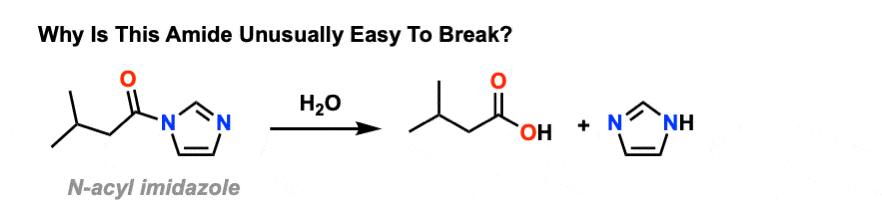
A mild alkaline hydrolysis of N- and NN-substituted amides and
Keywords: Alkaline hydrolysis amides |
|
Reactions of Anhydrides Mechanism of Anhydride Substitution
Nitriles are hydrolyzed with water in the presence of acid or base to yield carboxylic acids or carboxylate anions. • In this reaction the three C–N bonds are |
|
General Reaction of Anhydrides
leaving group the mechanism involves an additional proton transfer. Mechanisms for nitrile hydrolysis under acidic & basic conditions ... |
|
Chem 360 Jasperse Ch. 20 21 Notes + Answers. Carboxylic Acids
Access: Alkyl or Aryl Acids. • Alkyl group can be 1º 2º |
|
Chapter 21: Carboxylic Acid Derivatives
The large boiling points of nitriles acids and mechanism |
|
Hydrolysis of Nitriles
Hydrolysis of nitriles resembles the hydrolysis of amides The reaction is In basic solution the carboxylic acid product Mechanism of Hydrolysis of Nitriles |
|
Carboxylic Compounds, Nitriles, and Their Interconversion
Bruckner R (author), Harmata M (editor) In: Organic Mechanisms – Reactions, ditions (transformation A → B) and a total nitrile hydrolysis under basic |
|
88 Chapter 20: Carboxylic Acids and Nitriles 201 Naming
Acid or basic hydrolysis of a nitrile (mechanism, Fig 20 4) cyanide ion is an excellent nucleophile and will react with 1° and 2° alkyl halides and tosylates to give nitriles This reaction add one carbon |
|
217 HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES
Although nitriles are not carbonyl compounds, the C'N bond behaves chemically much The mechanism of ester saponification involves the reaction of the |
|
General Reaction of Anhydrides
The mechanism is similar to acid-catalyzed esterification/hydrolysis • Esters can of Nitriles • Mechanisms for nitrile hydrolysis under acidic basic conditions |