basic hydrolysis of nitriles
How nitrile is converted into a carboxylic acid?
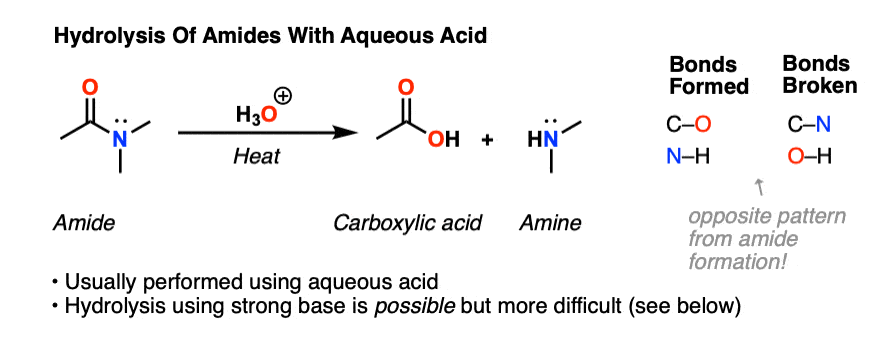
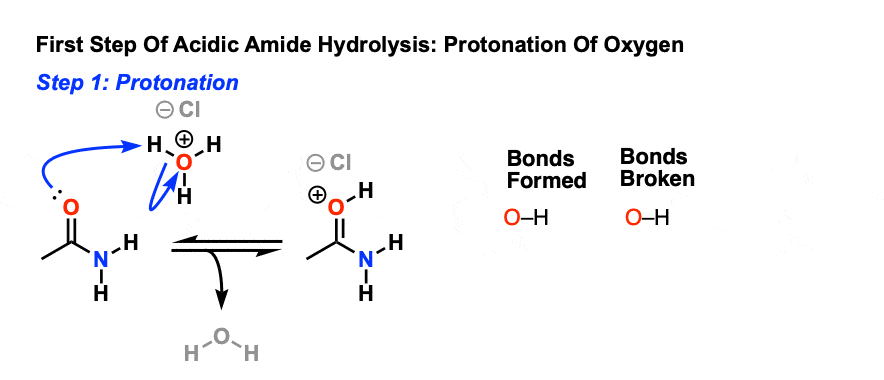
In both cases, the transformation consists of two main parts; conversion of the nitrile to an amide and hydrolysis of the amide to the corresponding carboxylic acid. In the first step of acid-catalyzed hydrolysis, the protonation of the nitrogen activates the C-N triple bond for a nucleophilic attack of water:
How does nitrile react with hydrochloric acid?
The nitrile is heated under reflux with dilute hydrochloric acid. Instead of getting an ammonium salt as you would do if the reaction only involved water, you produce the free carboxylic acid. For example, with ethanenitrile and hydrochloric acid you would get ethanoic acid and ammonium chloride.
How is nitrile hydrolyzed?
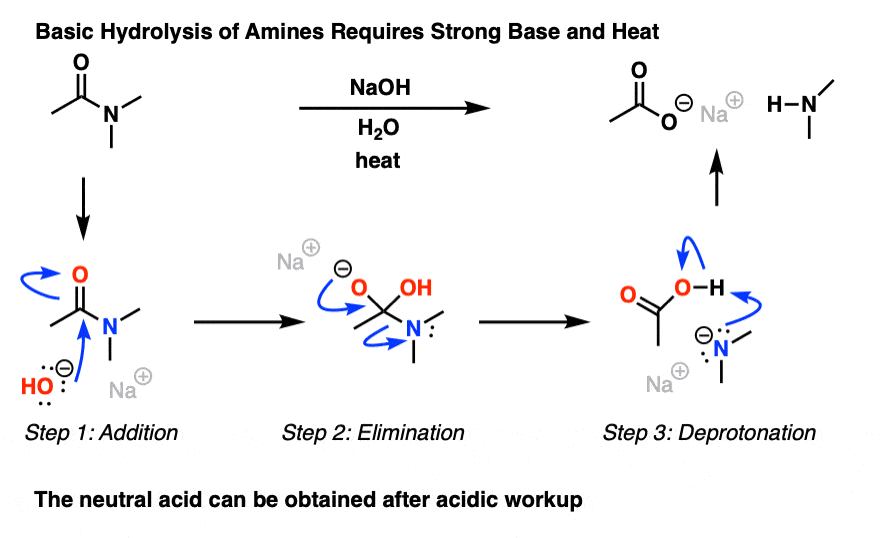
Mechanism for the basic hydrolysis of a nitrile to yield an amide, which is then hydrolyzed further to a carboxylic acid anion. The further hydrolysis of the amide intermediate takes place by a nucleophilic addition of hydroxide ion to the amide carbonyl group, which yields a tetrahedral alkoxide ion.
How does protonation affect nitrile hydrolysis?
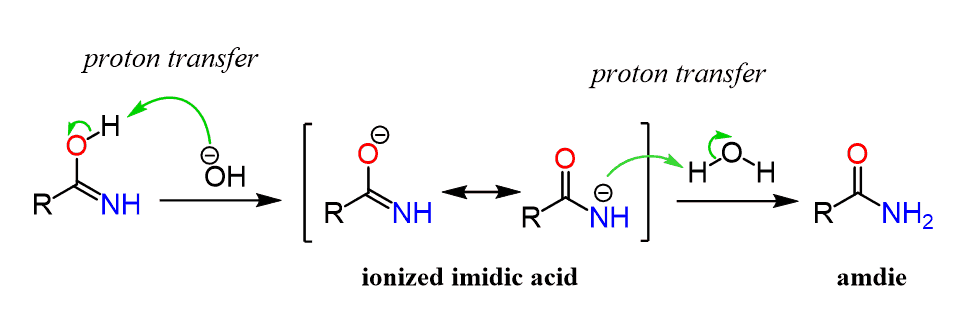
Protonation increases the electrophilicity of the nitrile so that it will accept water, a poor nucleophile. With base catalyzed hydrolysis, the strongly nucleophilic hydroxide anion is capable of directl addition to the carbon-nitrogen triple bond. During both mechanisms an amide intermediate is formed which usually is not isolated.

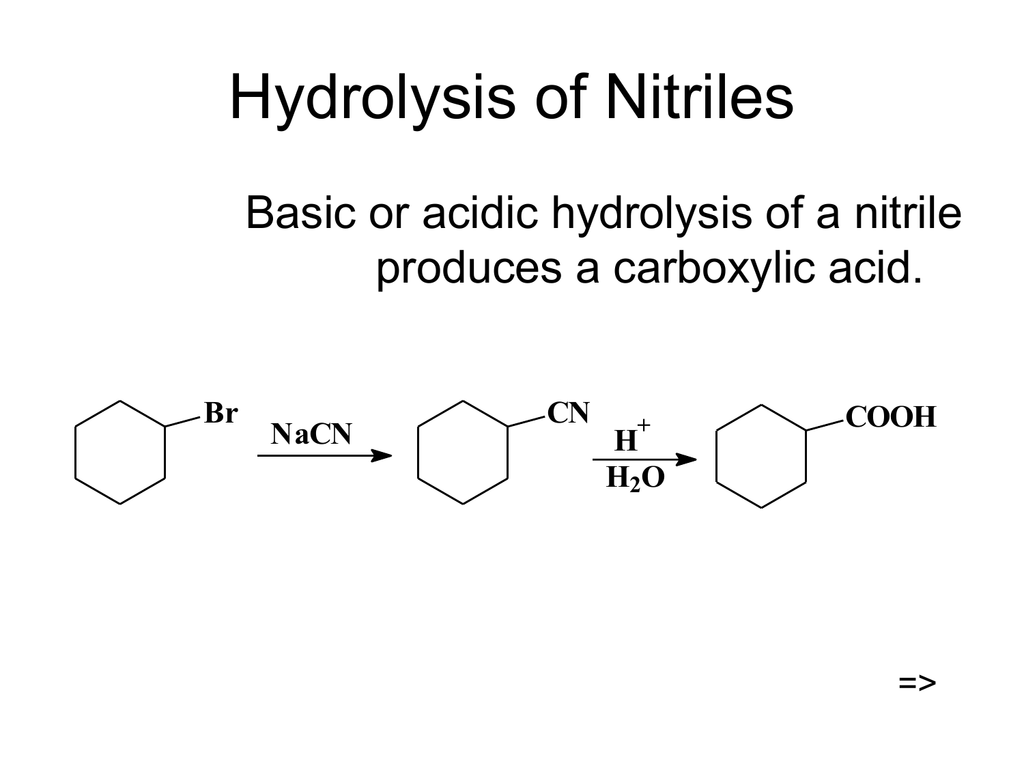
Hydrolysis of Nitriles

Hydrolysis of Nitriles

Hydrolysis of Nitriles
|
A mild alkaline hydrolysis of N- and NN-substituted amides and
Nitriles are hydrolyzed first to amides and further to carboxylic acids and ammonia with even more strong reaction conditions. The hydrolysis of amides and |
|
Dry hydrolysis of nitriles by sodium perborate and copper salts in
The multiphase reaction can be carried out in the absence of solvent water |
|
Base hydrolysis of coordinated acetonitrile
nitrogen atom. The metal ion promoted hydrolysis of nitriles has been studied for several nitriles.1-3 In |
|
CHEMOSELECTIVE HYDROLYSIS OF NITRILES USING
The reactions can be carried out in the absence of solvent water |
|
Stereo-electronic Effects in the Alkaline Hydrolysis of Benzamides
(Pseudo)-first-order rate constants for the alkaline hydrolysis of ¿-substituted benzonitriles and benzamides are reported. k h. Values for ki in the |
|
Mild and Efficient Conversion of Nitriles to Amides with Basic Urea
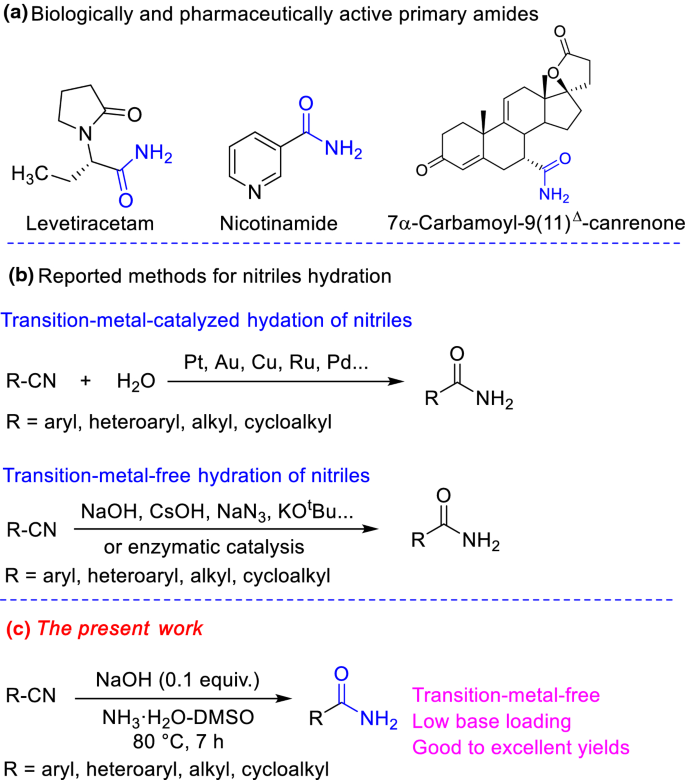
The hydrolysis of nitriles is a method frequently used for preparation of carboxylic acid amides. Traditional methods for the hydration of nitriles apply |
|
Assisted hydrolysis of the nitrile group of 2-aminoadamantane-2
The most obvious was the aldehyde- or ketone-assisted hydrolysis of the a-aminonitrile in alkaline solution to give the Schiff base (e.g. 8) of the a-aminoamide. |
|
Reactivity of coordinated nitriles
Kinetics of Base Hydrolysis of [Co(NH3)5(N==CCH2G^N)](CI04)3. A solution of the complex in 1 M NaC104 (ca. 8 X 10"4 M) was main-. |
|
The Acid-Catalyzed and Uncatalyzed Hydrolysis of Nitriles on
surface hydroxyl groups serving as the source of water. One of the best methods to synthesize carboxylic acids is the hydrolysis (acid- or base-catalyzed) of |
|
Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
From esters. Acidic hydrolysis of esters gives directly carboxylic acids while basic and alkenes by hydrolysis of nitriles and by treatment of Grignard ... |
|
A mild alkaline hydrolysis of N- and NN-substituted amides and
In general nitriles and amides are exceptionally stable to acid and basic hydrolysis and classically they are hydrolyzed under vigorous reaction conditions |
|
Dry hydrolysis of nitriles by sodium perborate and copper salts in
The multiphase reaction can be carried out in the absence of solvent water |
|
Mild and Efficient Conversion of Nitriles to Amides with Basic Urea
The hydrolysis of nitriles is a method frequently used for preparation of carboxylic acid amides. Traditional methods for the hydration of nitriles apply |
|
20_18_20.html.ppt [Read-Only]
Hydrolysis of nitriles resembles the hydrolysis of amides. In basic solution the carboxylic acid product ... Example: Acid Hydrolysis. (92-95%). |
|
The Hydrolysis of Nitriles with Acids
with physical properties of these acid solutions.1. One of the most interesting of these reactions was the hydrolysis of hydrogen cyanide with hydro-. |
|
Base hydrolysis of coordinated acetonitrile
of a deprotonated ammine on the nitrile group to pro- duce coordinated acetamidine. The present paper reports the investigation of the base hydrolysis of. |
|
Base hydrolysis of coordinated acetonitrile
of a deprotonated ammine on the nitrile group to pro- duce coordinated acetamidine. The present paper reports the investigation of the base hydrolysis of. |
|
KINETICS OF THE ALKALINE HYDROLYSIS OF PROPIONITRILE
acid and ammonia: Nitrile I4 amide I9 acid + ammonia. The existing state of knowledge of the reaction in alkaline solutions is unsatis-. |
|
Amide Synthesis through Selective Partial Hydrolysis of Nitriles in
Amide Synthesis through Selective Partial Hydrolysis of. Nitriles in Alkaline Media. Raymundo Yáñez-Alarid Elvira Santos-Santos and Eva F. Lejarazo-Gómez. |
|
Kinetic study of the base-catalyzed hydrolysis of aminocapronitrile
in methanol/water mixtures. This finding was explained by a single reaction mechanism similar to that for nitrile hydrolysis |
|
Hydrolysis of Nitriles
Hydrolysis of nitriles resembles the hydrolysis of amides The reaction is irreversible In basic solution the carboxylic acid product Example: Acid Hydrolysis |
|
Carboxylic Compounds, Nitriles, and Their Interconversion
Partial hydrolysis of a nitrile to a primary carboxylic acid amide under acidic condi - tions initiating the two-step total hydrolysis of acetone cyanohydrin (B) Grey |
|
The Acid-Catalyzed and Uncatalyzed Hydrolysis of Nitriles on
One of the best methods to synthesize carboxylic acids is the hydrolysis (acid- or base-catalyzed) of nitriles which occurs in two steps through the intermediate |
|
88 Chapter 20: Carboxylic Acids and Nitriles 201 Naming
Acid or basic hydrolysis of a nitrile (mechanism, Fig 20 4) cyanide ion is an excellent nucleophile and will react with 1° and 2° alkyl halides and tosylates to give nitriles This reaction add one carbon |



![PDF) Hydrolysis of [ 14 C]-Nitrile using Nitrilase (NIT) Biocatalysts PDF) Hydrolysis of [ 14 C]-Nitrile using Nitrilase (NIT) Biocatalysts](https://ars.els-cdn.com/content/image/3-s2.0-B9780080523491001177-gr55.gif)











![Carboxylic Acid \u0026 Nitriles - [PDF Document] Carboxylic Acid \u0026 Nitriles - [PDF Document]](https://ars.els-cdn.com/content/image/1-s2.0-S0040403914008399-gr1.jpg)


