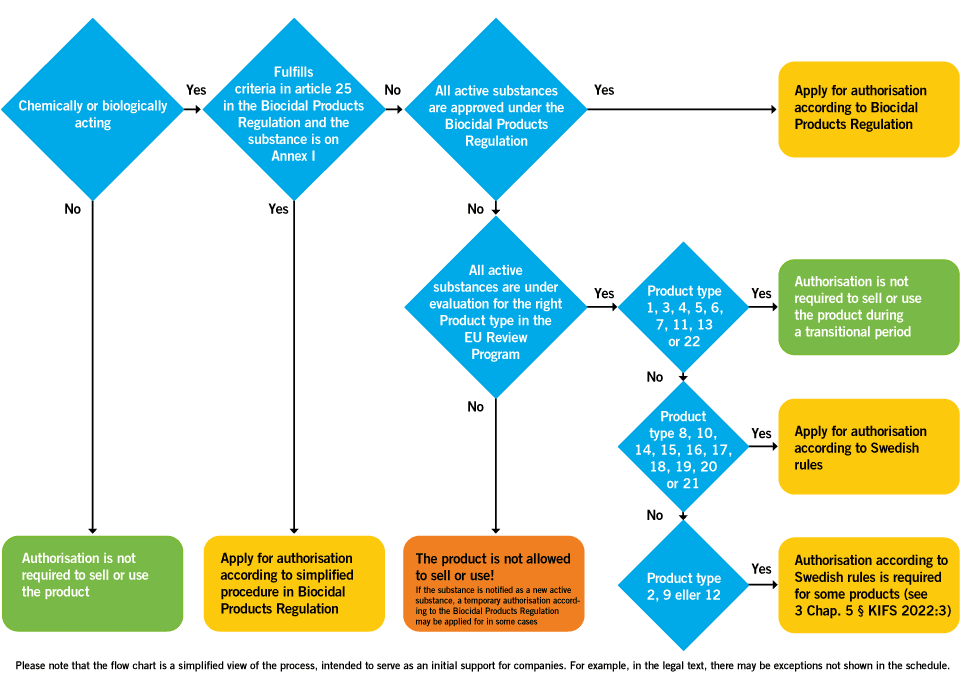
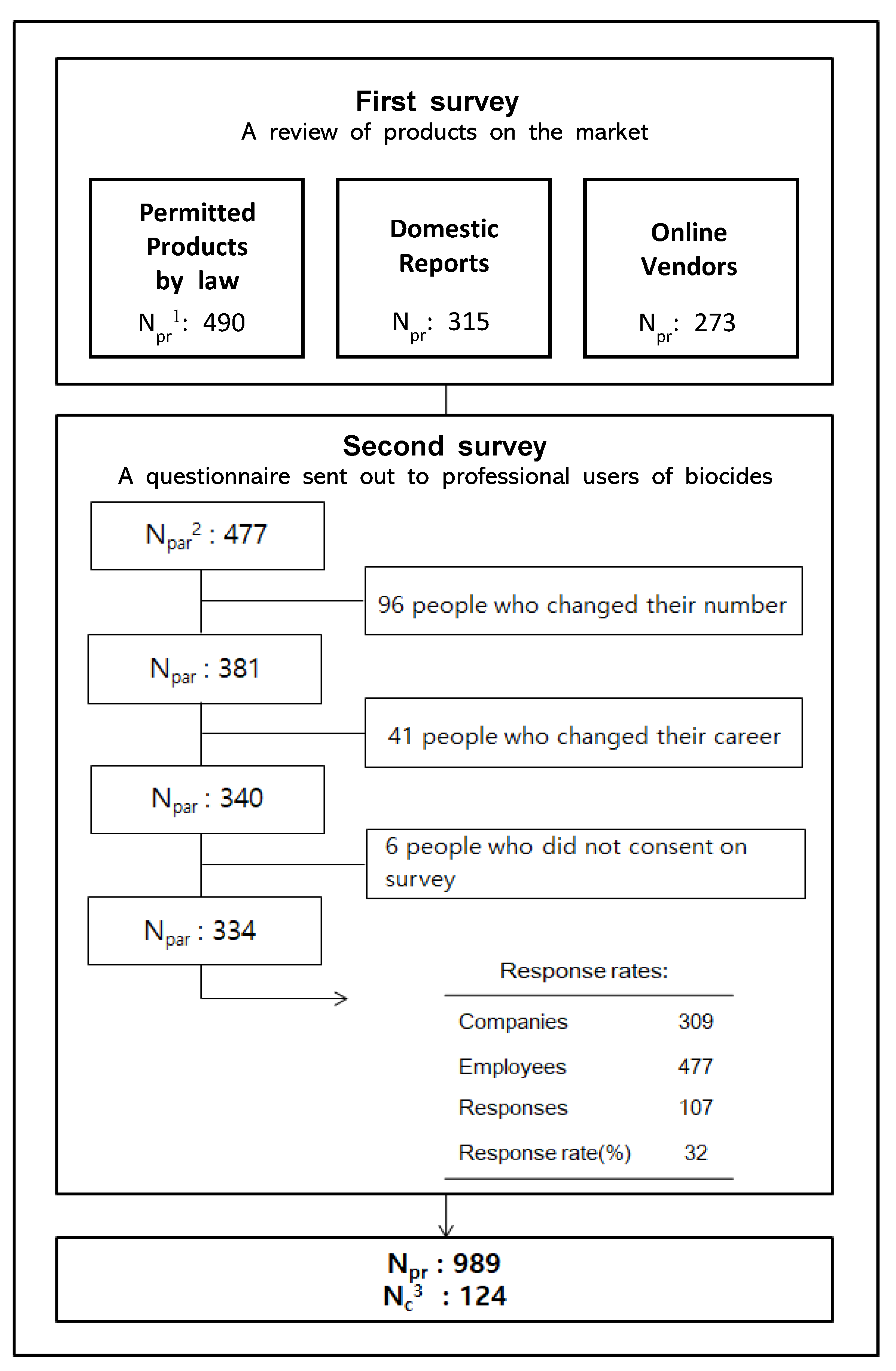
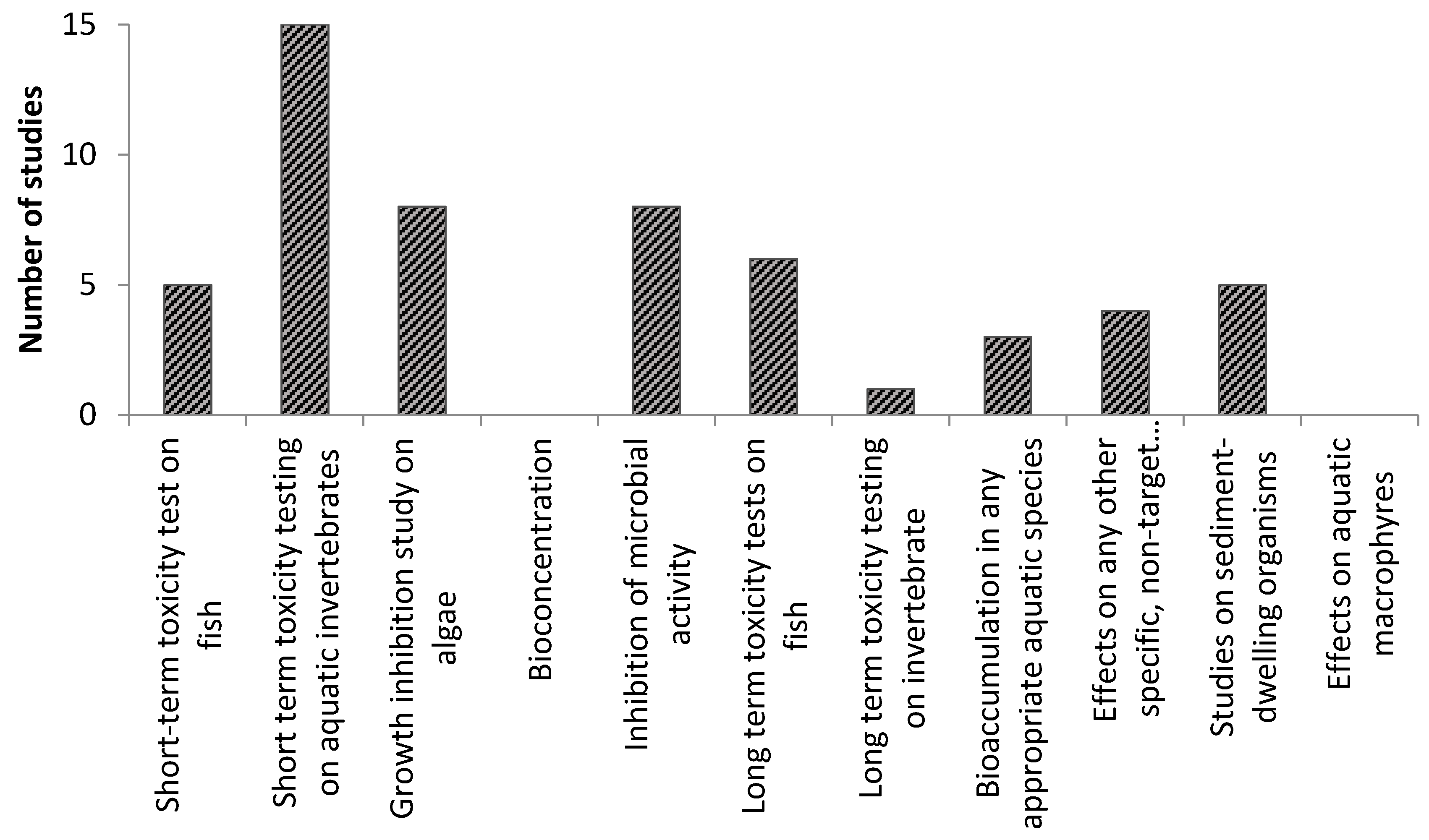
biocidal products regulation
What is a biocidal product authorisation?
1. An authorisation shall stipulate the terms and conditions relating to the making available on the market and use of the single biocidal product or the biocidal product family and include a summary of the biocidal product characteristics. 2.
What is the Biocidal Products Regulation (BPR)?
The Biocidal Products Regulation (BPR, Regulation (EU) 528/2012) concerns the placing on the market and use of biocidal products, which are used to protect humans, animals, materials or articles against harmful organisms like pests or bacteria, by the action of the active substances contained in the biocidal product.
What is 94 guidance on Biocidal Products Regulation?
94 Guidance on the Biocidal Products Regulation, Volume III: Human health, Part A: Information requirements investigated (e.g. tests carried out according to outdated guidelines), and in such cases, any adversity observed in parameters considered ‘sensitive to, but not diagnostic of, EATS’, cannot be dismissed.
What are the requirements for biocidal products?
40 Guidance on the Biocidal Products Regulation, Volume III: Human health, Part A: Information requirements • an intravenous dose (preferably), or if available, a single oral dose with assessment of biliary excretion (low dose level); and • a repeated dose. When intravenous dosing is not feasible, a justification should be provided.
Report on The Implementation of Regulation (EU) No 528/2012
According to Article 65(4), the European Commission is required to prepare every five years a report to the European Parliament and to the Council on the implementation of the Regulation. This report should be based on the reports provided by Member States. The first report was published in June 2021. health.ec.europa.eu
Article 2
The regulation outlines the general principles and products which fall under the scope of the biocidal products regulation. This includes mixtures, articles and materials treated with biocidal products, including furniture and textiles, as well as a provision on dual use covering biocidal products that have a dual function. health.ec.europa.eu
Article 3
This outlines the applicable definitions, making a distinction between those substances, mixtures and articles that should be regarded as biocidal products and those which should not, differentiating notably between articles that are biocidal products and those that are treated. health.ec.europa.eu
Animal Testing
Although the Regulation does not ban animal testing completely, it attempts to minimise it as much as possible. Article 62 introduces an obligation to share data on vertebrate animal tests in exchange for fair compensation, and a prohibition to duplicate such tests, which is aimed at saving costs, as well as animal lives. It also encourages data-sh
How Are Products Authorised, and by Whom?
To obtain the authorisation needed to supply and use these products, companies must demonstrate that the product is effective and does not present unacceptable risks to humans, animals or the environment. Individual EU countries are responsible for authorising biocidal products that are made available on their own territory with mutual recognition
Role of ECHA
The European Chemicals Agency (ECHA) is responsible for providing technical and scientific support in implementing Regulation (EU) 528/2012. Through its Biocidal Product Committee, it provides opinions to the European Commission on: 1. Approving active substances 2. Authorising biocidal productsat EU level 3. Various other scientific and technical
Expert Group and Standing Committee
An expert group, made up of representatives of the competent authorities for the implementation of the Biocidal Products Regulation, assists the Commission with the preparation of policy initiatives, delegated acts, and implementation of the Biocidal Products Regulation, including the coordination of certain Member States’ activities. Representativ
Competent Authorities
The list of competent authorities, helpdesks and stakeholders for the implementation of the Regulation is available here. health.ec.europa.eu

Tips to successfully comply with the Biocidal Product Regulation

UK Biocidal Products Regulation in a nutshell (webinar)

Regulation of Biocides in UK
|
Regulation (EU) No 528/2012 of the European Parliament and of the
22 mai 2012 with this Regulation. Treated articles should not be placed on the market unless all active substances contained in the biocidal products ... |
|
B REGULATION (EU) No 528/2012 OF THE EUROPEAN
25 avr. 2014 (a) the establishment at Union level of a list of active substances which may be used in biocidal products;. (b) the authorisation of biocidal ... |
|
B REGULATION (EU) No 528/2012 OF THE EUROPEAN
20 nov. 2019 1. This Regulation shall apply to biocidal products and treated articles. A list of the types of biocidal products covered by this Regu-. |
|
Guidance on the Biocidal Products Regulation
An applicant for authorisation of a biocidal product must propose classification labelling and packaging which complies with the CLP Regulation. Directive 67/ |
|
L 167 Official Journal
22 mai 2012 Regulation (EU) No 528/2012 of the European Parliament and of the Council of ... This Regulation should apply to biocidal products that in. |
|
Guidance on the Biocidal Products Regulation - Volume II Efficacy
1 févr. 2022 The Guidance on the Biocidal Products Regulation (BPR) is to be applied to applications for active substance approval and product authorisation ... |
|
Guidance on the Biocidal Products Regulation - Volume V
After the Biocides Product Regulation (BPR Regulation (EU) 528/2012) came into force and the biocides assessment had moved to the European Chemicals Agency |
|
Practical Guide on Biocidal Products Regulation
The basic principle in the Biocidal Products Regulation ((EU) No 528/2012. (BPR)) is that a biocidal product (BP) must be authorised before it can be made |
|
Guidance on the Biocidal Products Regulation Volume II Efficacy
Product 10 - 40 The Guidance on the Biocidal Products Regulation (BPR) is to be applied to applications for active substance approval and product authorisation ... |
|
COMMISSION IMPLEMENTING DECISION (EU) 2021/1283 of 2
2 août 2021 on the non-approval of certain active substances in biocidal products pursuant to Regulation (EU). No 528/2012 of the European Parliament ... |
|
Guidance on the Biocidal Products Regulation - ECHA - europaeu
In addition to the BPR guidance, the Biocidal Products Directive (BPD) guidance and other related documents are still considered applicable for new submissions |
|
The EU Biocidal Products Regulation - AeroSpace and Defence
1 0 What is the EU Biocidal Products Regulation (BPR)? The BPR1 is an EU regulation concerning the regulation of biocidal products; those products designed to |
|
Download the EDANA Guide to Biocidal Product regulation
Biocidal Products Regulation (BPR) Developed and published by EDANA March 2015 Disclaimer: This document intends to provide guidance to EDANA's |
|
Ozone as active substance under the Biocidal Products Regulation
Ozone is declared an active substance under the EU Biocidal Products Regulation No 528/2012 (BPR) starting as of September 1st, 2013 In effect the BPR is |
|
Biocidal Products
L N 348 of 2013 PESTICIDES CONTROL ACT (CAP 430) Biocidal Products ( Implementation of Regulation (EU) No 528/2012) Regulations, 2013 |
|
Biocides and the REACH and CLP Regulations Introduction
The Biocidal Products Regulation, No 528/2012 (“BPR”) took effect on 1 September 2013 and repeals the previous Biocidal Products Directive (Directive 98/8/EC) |
|
Biocidal Products Regulation Whitepaper - Contec, Inc
Biocidal products have been regulated in the European Union (EU) by the EU Biocidal Product Regulation 528/2012 (BPR) since 1 September 2013 The aim |
|
Impact of the European Biocidal Products Regulation - ProMinent
Unlike the previous biocidal products legislation, so-called “in-situ” procedures such as electrolysis, chlorine dioxide and ozone now also fall under this |
![ECHA Guidance on the Biocidal Products Regulation - [PDF Document] ECHA Guidance on the Biocidal Products Regulation - [PDF Document]](https://echa.europa.eu/documents/10162/14727420/same_biocidal_product_graphic_en.png/53f91049-11b8-4ee6-b2ac-385befc5f2b3?t\u003d1477554844739)