ich q7
|
Q7 Good Manufacturing Practice Guidance for Active
This revision changes the ICH codification from Q7A to Q7. This revision also adds the ICH section numbers in parentheses at the end of each paragraph in. |
|
Ich harmonised tripartite guideline good manufacturing practice
Q7. Current Step 4 version dated 10 November 2000. This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to |
|
ICH guideline Q7 on good manufacturing practice for active
23 июл. 2015 г. ICH Q7 also describes principles of GMPs to be applied in the manufacture of APIs for use in clinical trials (section 19) and for APIs ... |
|
Q 7 Good Manufacturing Practice for Active Pharmaceutical
Impurity profiles are normally not necessary for APIs from herbal or animal tissue origin. Biotechnology considerations are covered in ICH Guideline Q6B. 11.22 |
|
Q7 Good Manufacturing Practice Guidance for Active
2.4: Does ICH Q7 expect that sampling be performed by the quality unit? No. ICH Q7 does not prescribe specifically who should perform the sampling (ICH Q7. |
|
Q7 Implementation Working Group ICH Q7 Guideline: Good
10 июн. 2015 г. ICH Q7 also describes principles of GMPs to be applied in the manufacture of APIs for use in clinical trials (Section 19) and for APIs ... |
|
ICH guideline Q10 on pharmaceutical quality system - Step 5
Relationship of ICH Q10 to regional GMP requirements ISO standards and ICH Q7 ... 5. 1.4. Relationship of ICH Q10 to regulatory approaches ... |
|
Q7 Implementation Working Group ICH Q7 Guideline: Good
10 июн. 2015 г. ICH Q7 also describes principles of GMPs to be applied in the manufacture of APIs for use in clinical trials (Section 19) and for APIs ... |
|
ICH Q7 Training Chapter 19: APIs for Use in Clinical Trials
APIs For Use In Clinical Trials. • Why is there a special section on APIs for clinical use? - Processes and controls change during development. |
|
Ich harmonised tripartite guideline good manufacturing practice
Q7. Current Step 4 version dated 10 November 2000. This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to |
|
Q7 Good Manufacturing Practice Guidance for Active
This revision changes the ICH codification from Q7A to Q7. This revision also adds the ICH section numbers in parentheses at the end of each paragraph in. |
|
Ich harmonised tripartite guideline good manufacturing practice
Q7A. Approval by the Steering Committee under Step 4 and recommendation for adoption to the three ICH regulatory bodies. 10. November. 2000. Q7 |
|
Q7 Good Manufacturing Practice Guidance for Active
Since the ICH Q7 Guidance was finalized experience with implementing the 1.1: Should GMP according to ICH Q7 be applied for manufacturing steps before ... |
|
ICH guideline Q7 on good manufacturing practice for active
23-Jul-2015 ICH Q7 also describes principles of GMPs to be applied in the manufacture of APIs for use in clinical trials (section 19) and for APIs ... |
|
ICH Topic Q 7 Good Manufacturing Practice for Active
CPMP/ICH/4106/00. ICH Topic Q 7. Good Manufacturing Practice for Active Pharmaceutical Ingredients. Step 5. NOTE FOR GUIDANCE ON GOOD MANUFACTURING PRACTICE |
|
Ich harmonised tripartite guideline good manufacturing practice
Q7. Current Step 4 version. Q7A. Approval by the Steering Committee under Step 4 and recommendation for adoption to the three ICH regulatory bodies. |
|
1 Final Concept Paper ICH Q7 Q&As Q7: Good Manufacturing
17-Oct-2012 The ICH Q7 Guideline is implemented successfully in the regulatory framework by WHO and most authorities around the world. However experience ... |
|
Q7 Implementation Working Group ICH Q7 Guideline: Good
10-Jun-2015 ICH Q7 Guideline: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients. Questions and Answers. Current version. |
|
Q&A on ICH Q7 – Good Manufacturing Practice Questions and
21-Aug-2017 Importance of the ICH Q7 Guideline. • First internationally harmonized Good Manufacturing. Practice (GMP) guidance developed jointly by ... |
|
ICH guideline Q10 on pharmaceutical quality system - Step 5
recommendation for adoption to the three ICH regulatory bodies. Regional GMP requirements the ICH Q7 Guideline |
|
Q7 Good Manufacturing Practice Guidance for Active
ICH HARMONISED TRIPARTITE GUIDELINE GOOD MANUFACTURING PRACTICE GUIDE FOR ACTIVE PHARMACEUTICAL INGREDIENTS Q7 Current Step 4 version dated 10 November 2000 This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties in accordance with the ICH Process |
| Q 7 Good Manufacturing Practice for Active Pharmaceutical |
What is the ICH guidance Q7 Good Manufacturing Practice Guidance for active pharmaceutical ingredients?
- The ICH guidance Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality.
What is ICH Q10?
- ICH Q10 describes one comprehensive model for an effective pharmaceutical quality system that is based on International Standards Organisation (ISO) quality concepts, includes applicable Good Manufacturing Practice (GMP) regulations and complements ICH Q8 “Pharmaceutical Development” and ICH Q9 “Quality Risk Management”.
How is equipment cleaning addressed in ICH Q7?
- Equipment cleaning is addressed in two sections in ICH Q7. Although the cleaning validation (ICH Q7, section XII.G (12.7)) does not specifically address time limits for cleaning, ICH Q7, paragraph 5.21, indicates that the maximum time between completion of processing and equipment cleaning (dirty hold time) should be established by the company.
What is ICH Guideline Q5A?
- Monitoring of bioburden and, where needed, endotoxin levels at appropriate stages of production; and Viral safety concerns as described in ICH Guideline Q5A Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin.
|
Q7 Good Manufacturing Practice Guidance for Active - FDA
Active Pharmaceutical Ingredients This revision changes the ICH codification from Q7A to Q7 This revision also adds the ICH section numbers in parentheses |
|
Q7 Q&A - good manufacturing practice for active pharmaceutical
23 juil 2015 · ICH guideline Q7 on good manufacturing practice for active pharmaceutical ingredients – questions and answers EMA/CHMP/ICH/468930/ |
|
Interprétation of ICH Q7 document - APIC (CEFIC)
ACTIVE PHARMACEUTICAL INGREDIENTS COMMITTEE GMPs for APIs: “How to do” Document Interpretation of the ICH Q7 Guide Version 11 (Update |
|
ICH Q7 Training Courses 2021 - ICH Q7 Week
ICH Q7 Compliance for APIs Manufactured by Chemical Synthesis 21 - 23 June 2021 ICH Q7 in modern API Manufacturing – what to do and how to do |
|
Differences between GMP Part I and Part II (and ICH Q7) - Swissmedic
ICH Q7A Annex 18 EU GMP 2001 EU GMP Part I Part II Annexes 2005 2000 Various ICH guideline Q10 on pharmaceutical quality system 2008 I II |
|
IWG MEMBERS ON ICH Q7 Q&A AND Q11 STARTING MATERIALS
OPQ'S OUDERKIRK ON ICH Q7 Q&A ICH Q7 guidance, which is GMP for the manufacture of APIs, was the first internationally harmonized tripartite guidance |






























































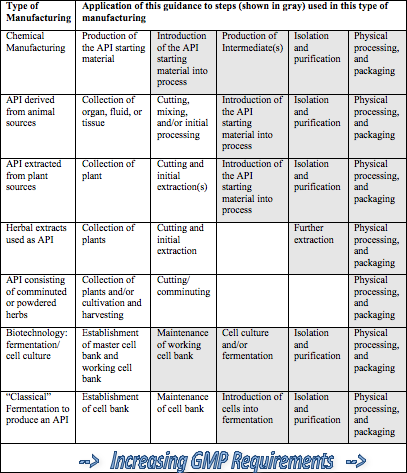







![Ich guidelines - [PPT Powerpoint] Ich guidelines - [PPT Powerpoint]](https://www.complianceiq.com/Images/Training/Details/Detailsbfe518f6-064d-4b41-a618-d44cee8494a6131281652965324734.jpg)