ich q8 pdf
|
Q8(R2)
2. (Pharmaceutical Development) for drug products as defined in the scope of Module 3 of the Common Technical Document (ICH guideline M4). The guideline does |
|
ICH Guideline Q8 (R2) on Pharmaceutical Development Step 5
22 Jun 2017 ICH guideline Q8 (R2) on pharmaceutical development. EMA/CHMP/ICH/167068/2004. Page 11/24. Formal experimental design: A structured organized ... |
|
Q8(R2): Pharmaceutical Development
D. National Health Laboratory Luxembourg. Ch i. ICH IWG Q8 Q9 Q10. Chair-person ICH IWG Q8 |
|
Q8(R2)
2. (Pharmaceutical Development) for drug products as defined in the scope of Module 3 of the Common Technical Document (ICH guideline M4). The guideline does |
|
ICH-Endorsed Guide for ICH Q8/Q9/Q10 Implementation Document
6 Dec 2011 The ICH Quality Implementation Working Group (Q-IWG) has prepared 'Points to. Consider' covering topics relevant to the implementation of ICH Q8 ... |
|
Quality Implementation Working Group on Q8 Q9 and Q10
15 Apr 2009 Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf approved Jun. 04 2008 ... are being marketed as 'ICH compliant solutions' or ICH Q8 |
|
ICH guideline Q8 Q9 and Q10 - questions and answers volume 4
http://www.ich.org/LOB/media/MEDIA1957.pdf approved Nov. 09 2005. ICH Q10 Under Quality by Design establishing a design space or using real time release ... |
|
ICH guideline Q9 on quality risk management – Step 5
Link to: ICH Q8/Q9/Q10 Training material. Link to: ICH Q8/Q9/Q10 Points to consider. Page 2. ICH guideline Q9 on quality risk management. EMA/CHMP/ICH/24235/ |
|
TECHNICAL AND REGULATORY CONSIDERATIONS FOR
19 Nov 2019 These guidelines are valuable in the assessment of Chemistry Manufacturing and Controls (CMC) changes across the product lifecycle. ICH Q8(R2) ... |
| ICH guideline Q11 on development and manufacture of drug |
|
Q8(R2)
ICH HARMONISED TRIPARTITE GUIDELINE. PHARMACEUTICAL DEVELOPMENT. Q8(R2). Current Step 4 version dated August 2009. This Guideline has been developed by the |
|
ICH guideline Q8 (R2) on pharmaceutical development - Step 5
22-Jun-2017 ICH guideline Q8 (R2) on pharmaceutical development. EMA/CHMP/ICH/167068/2004. Page 2/24. Document History. First. Codification. History. |
|
ICH-Endorsed Guide for ICH Q8/Q9/Q10 Implementation Document
06-Dec-2011 The ICH Quality Implementation Working Group (Q-IWG) has prepared 'Points to. Consider' covering topics relevant to the implementation of ICH Q8 ... |
|
Q8(R2): Pharmaceutical Development
release testing. ICH-GCG ASEAN Kuala Lumpur |
|
Q8(R2)
PHARMACEUTICAL DEVELOPMENT. Q8(R2). Current Step 4 version dated August 2009. This Guideline has been developed by the appropriate ICH Expert Working Group. |
|
ICH Q8: Pharmaceutical Development
ICH Q8: Pharmaceutical. Development. Pharmaceutical Quality Forum. November 2004. John C Berridge. Pfizer Global R&D. Sandwich. UK. Q8 Rapporteur (EFPIA) |
|
ICH guideline Q9 on quality risk management - Step 5
November 2005. Date for coming into effect. January 2006. Link to: ICH Q8/Q9/Q10 Training material. Link to: ICH Q8/Q9/Q10 Points to consider |
|
ICH guideline Q8 Q9 and Q10 - questions and answers volume 4
ICH Q10. Pharmaceutical Quality Systems http://www.ich.org/LOB/media/MEDIA3917.pdf approved Jun. 04 2008. ICH guideline Q8 Q9 and Q10 - questions and |
|
ICH guideline Q11 on development and manufacture of drug
discussed in ICH Q8 for drug product a greater understanding of the drug substance and its manufacturing process can create the basis for more flexible |
|
Q8(R2) - ICH
ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL DEVELOPMENT Q8(R2) Current Step 4 version dated August 2009 This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties in accordance with the ICH Process |
|
Pharmaceutical Development: ICH Q8/Q(8)R
ICH Q8 Guidance Provides guidance on the contents of Section 3 2 P 2 (Pharmaceutical Development) Describes good practices for pharmaceutical product development Introduces concepts of Design space Flexible regulatory approaches Quality Risk Management (Q9) Does not discuss QbD |
|
IMPURITIES GUIDELINE FOR RESIDUAL S Q3C(R8) - ICH
ICH HARMONISED GUIDELINE IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS Q3C(R8) Current Step 4 version dated 22 April 2021 This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties in accordance with the ICH Process At Step 4 |
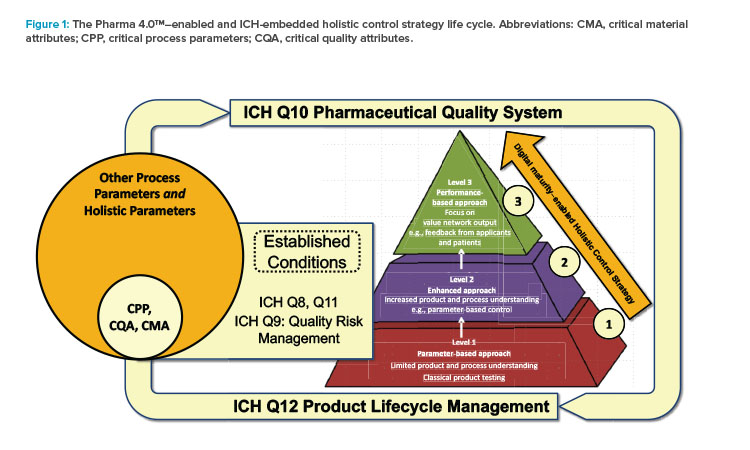
What is the ICH Q8 Pharmaceutical Development guideline?
- This guideline is an annex to ICH Q8 Pharmaceutical Development and provides further clarification of key concepts outlined in the core guideline. In addition, this annex describes the principles of quality by design1(QbD).
When did Ich recode the Q8 parent guidance?
- Following the addition of the annex to the Q8 parent guidance, ICH recoded the guidance Q8(R1). In August 2009, ICH issued Q8(R2) with corrected captions for figures 2a and 2b in Appendix 2, section C.
What is ICH Q10?
- Regional GMP requirements, the ICH guidance “Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients,” and ISO quality management system guidelines form the foundation for ICH Q10. To meet the objectives described below, ICH Q10 augments GMPs by describing specific quality system elements and management responsibilities.
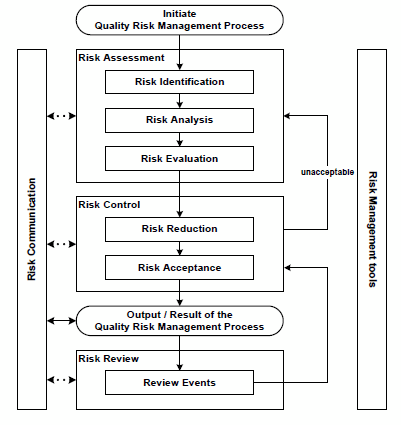
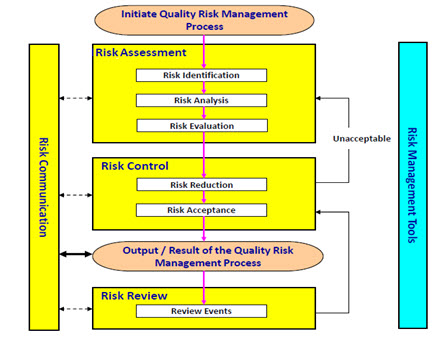
What is Ich Q9 quality risk management?
- Where a company chooses to apply quality by design and quality risk management (ICH Q9 Quality Risk Management), linked to an appropriate pharmaceutical quality system, opportunities arise to enhance science- and risk-based regulatory approaches (see ICH Q10 Pharmaceutical Quality System).6
|
Q8(R2) - ICH
ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL DEVELOPMENT Q8(R2) Current Step 4 version dated August 2009 This Guideline has |
|
ICH Q8 - Académie Nationale de Pharmacie
2 mar 2011 · De ce fait, les notes explicatives IQH Q8, Q9 et Q10 virent le jour ICH Quality Vision “Develop a harmonized pharmaceutical quality system |
|
La démarche Quality by design - Thesesante - Université Toulouse
La deuxième partie du l'ICH Q8(R2) détaille la démarche Quality by Design (QbD ) par la Pharmaceutical Quality System pdf [En ligne] Disponible sur : |
|
ICH Q8: Pharmaceutical Development
1 ICH Q8: Pharmaceutical Development Pharmaceutical Quality Forum November 2004 John C Berridge Pfizer Global R&D Sandwich UK Q8 Rapporteur |
|
Q8 (R2) - European Medicines Agency - europaeu
22 jui 2017 · Any changes between the proposed commercial formulation Page 8 ICH guideline Q8 (R2) on pharmaceutical development EMA/CHMP/ICH/ |
|
LICH Q8(R2): Dévelopment Pharmaceutique - Canadaca
10 fév 2016 · En adoptant cette ligne directrice de l'ICH, Santé Canada fait siens les in English under the following Title: ICH guidance document Q8(R2): |
|
ICH Q8/Q8R – Pharmaceutical Development - Drug Information
Key Points from ICH Q8 Core Document • Quality cannot be tested into products, it should be built in by design • Pharmaceutical development provides the |
|
Applying ICH Q8(R2), Q9, and Q10 Principles to Chemistry - FDA
20 nov 2009 · information regarding systematic approaches to quality risk management Page 2 MANUAL OF POLICIES AND PROCEDURES CENTER FOR |



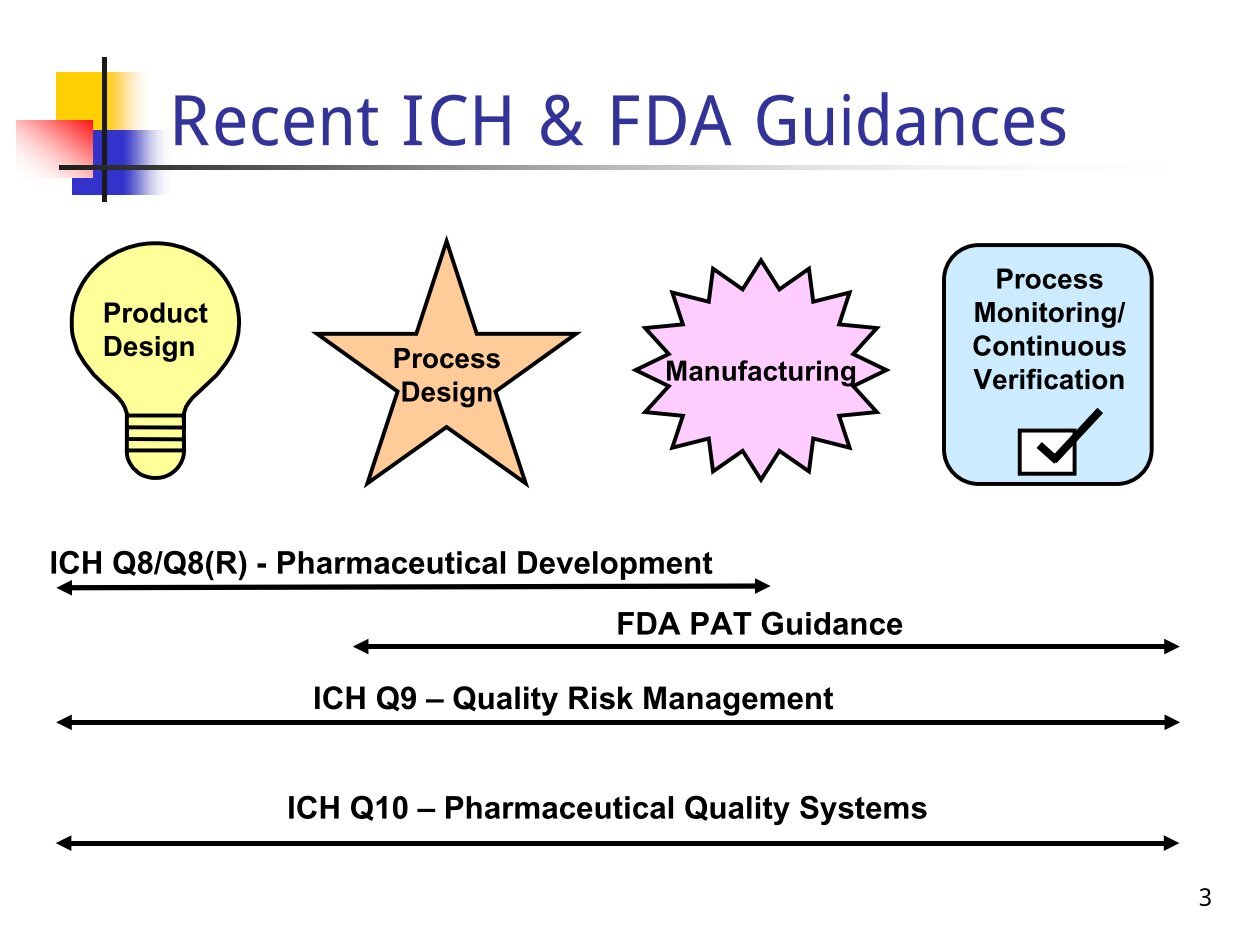






























![PDF] PQLI Application of Science- and Risk-based Approaches (ICH PDF] PQLI Application of Science- and Risk-based Approaches (ICH](http://docplayer.net/docs-images/58/41554386/images/2-0.png)










![PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a](https://europepmc.org/articles/PMC4070262/bin/12248_2014_9598_Fig2_HTML.jpg)




![PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a](https://www.gmp-compliance.org/files/eca/userImages/training.img/Z-ECA-Webinar-ICHQ12-New-approach.jpg)
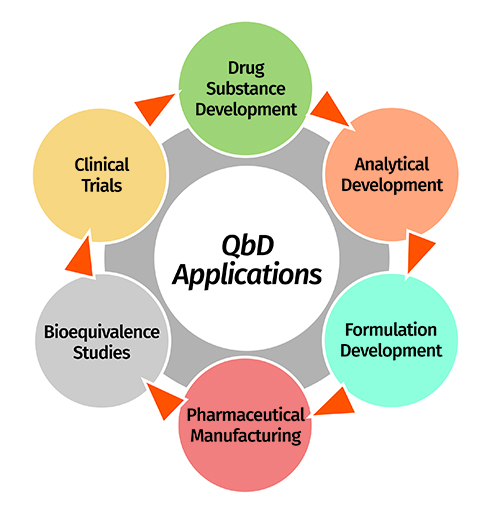






![Full text] Quality by design approaches for topical dermatological Full text] Quality by design approaches for topical dermatological](https://demo.vdocuments.site/img/378x509/reader018/reader/2020021822/5868bb1c1a28abf52b8b4d6f/r-2.jpg)
![ICH Q8 (R2) - [PDF Document] ICH Q8 (R2) - [PDF Document]](https://i1.rgstatic.net/publication/326595252_QUALITY_BY_DESIGN-_A_NEW_APPROACH_TO_DRUG_DEVELOPMENT/links/5b587970a6fdccf0b2f3bb2e/largepreview.png)














![Full text] Quality by design approaches for topical dermatological Full text] Quality by design approaches for topical dermatological](https://www.gmp-compliance.org/files/eca/userImages/training.img/Z-ECA-ICH-Q7-Courses-2017-December.jpg)

