ich q9 pdf
|
ICH guideline Q9 on quality risk management - Step 5
EMA/CHMP/ICH/24235/2006. Committee for Human Medicinal Products. ICH guideline Q9 on quality risk management. Step 5. Transmission to CHMP. June 2005. |
|
ICH E10
ICH HARMONISED TRIPARTITE GUIDELINE. QUALITY RISK MANAGEMENT. Q9. Current Step 4 version dated 9 November 2005. This Guideline has been developed by the |
|
ICH guideline Q9 (R1) on quality risk management
18 nov 2021 16 December 2021. EMA/CHMP/ICH/24235/2006. Committee for Medicinal Products for Human Use. ICH guideline Q9 (R1) on quality risk management. |
|
Fernando Tazón
ICH Q 9. •Prevención de Riesgos de Calidad (PRC). Quality Risk Management (QRM). • Recomendado para adopción en el Step 4 del Proceso de ICH el 9. |
|
QUALITY RISK MANAGEMENT
9 nov 2005 ICH HARMONISED TRIPARTITE GUIDELINE. QUALITY RISK MANAGEMENT. Q9 ... recommendation for adoption to the three ICH regulatory bodies. |
|
Quality Risk Management ICH Q9(R1)
26 nov 2021 The ICH Q9 Guideline has been revised to address the following: •. The QRM principles and framework of ICH Q9 have been instrumental in ... |
|
Quality Risk Management ICH Q9
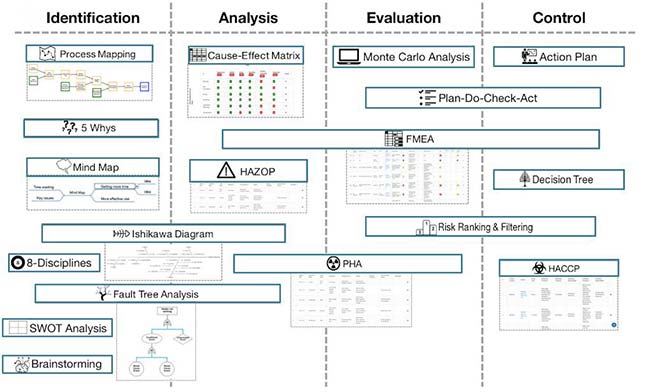
http://www.sverdrup.com/safety/fta.pdf. ICH Q9. Annex I: Methods & Tools prepared by some members of the ICH Q9 EWG for example only; not an official |
|
APLICACIÓN DE LA CALIDAD INTEGRAL AL MEDICAMENTO
ICH Q8. ICH. Q11. GLP. GCP. GMP. ICH Q7. ICH Q9. ICH ICH Q10. Evaluación de riesgos según. ICH Q9. “Espacio de diseño” ... Manual del investigador. |
|
ICH guideline Q10 on pharmaceutical quality system - Step 5
ICH Q9 “Quality Risk Management”. ICH Q10 is a model for a pharmaceutical quality system that can be implemented throughout the different stages of a |
|
ICH-Endorsed Guide for ICH Q8/Q9/Q10 Implementation Document
6 dic 2011 The introduction of ICH Q9 states that: “…the protection of the patient by managing the risk to quality should be considered of prime importance ... |
|
QUALITY RISK MANAGEMENT Q9(R1) - ICH
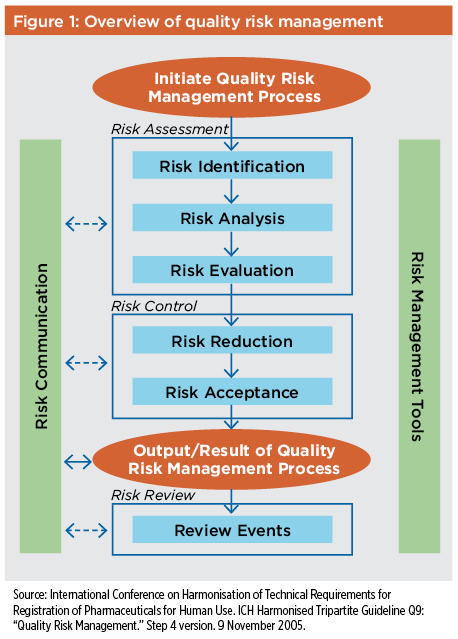
ICH Q9(R1) Guideline 1 1 1 INTRODUCTION 2 Risk management principles are effectively utilized in many areas of business and government 3 including finance insurance occupational safety public health pharmacovigilance and by 4 agencies regulating these industries In the pharmaceutical sector the principles and framework |
|
QUALITY RISK MANAGEMENT Q9(R1) - databaseichorg
ICH HARMONISED GUIDELINE QUALITY RISK MANAGEMENT Q9(R1) Final version Adopted on 18 January 2023 This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties in accordance with the ICH Process At Step 4 |
|
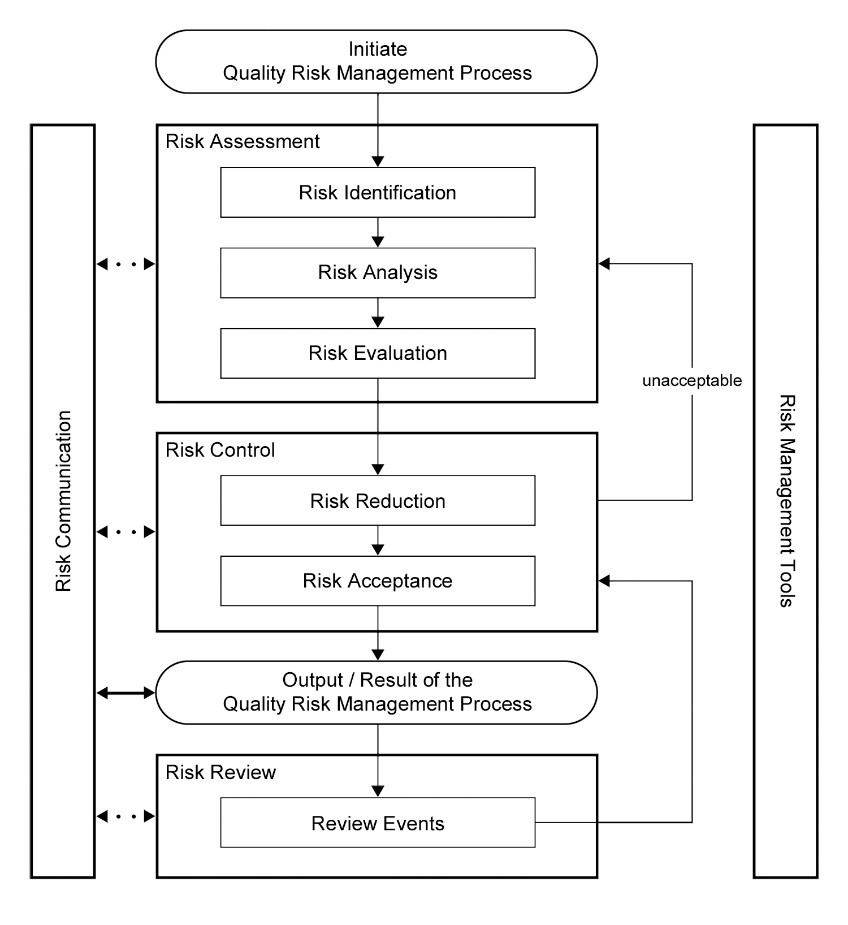
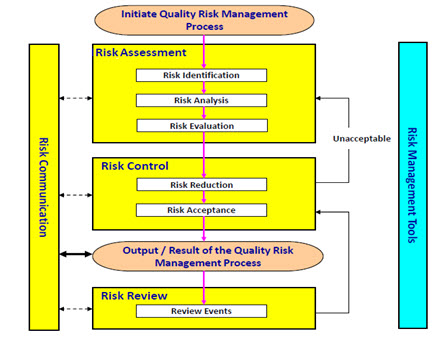
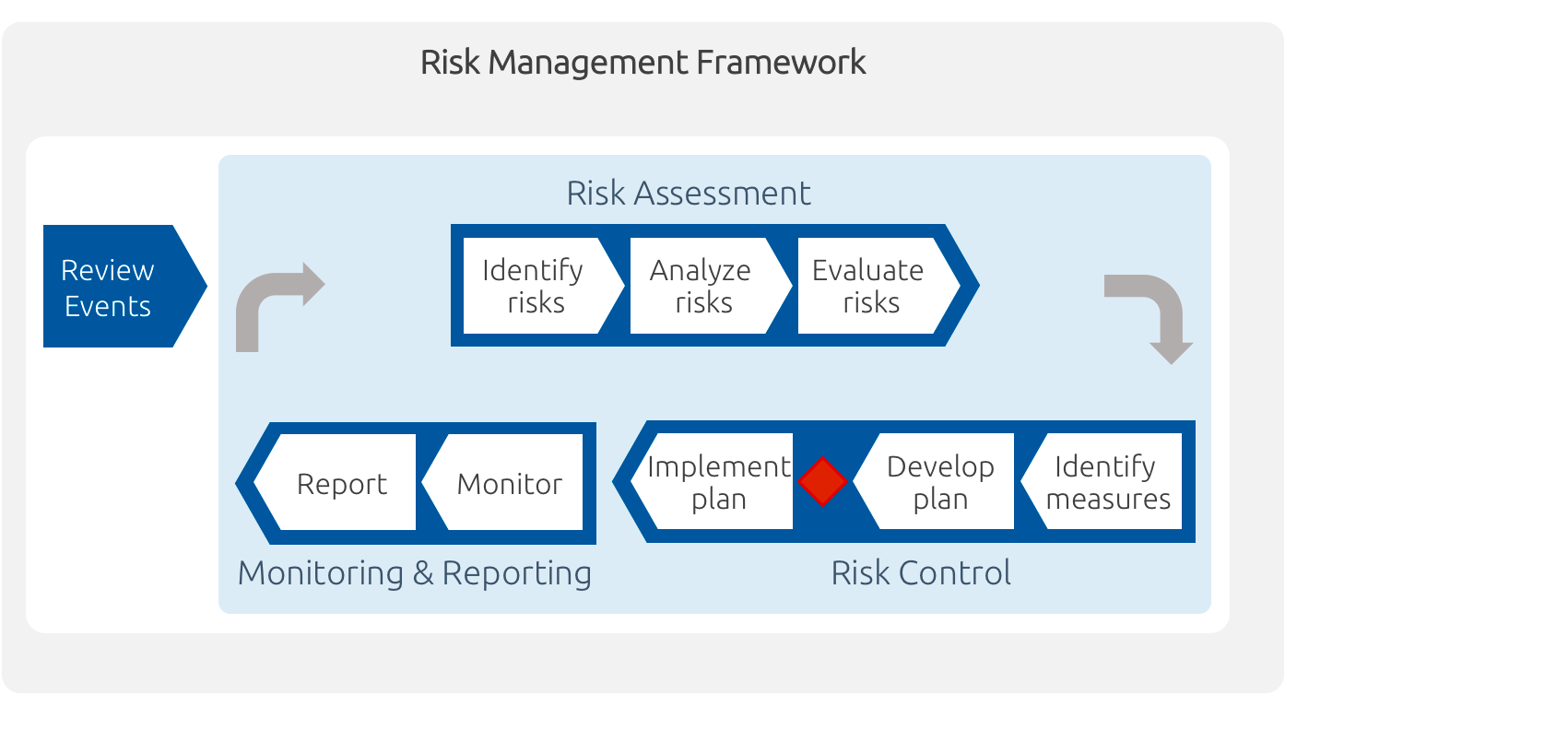
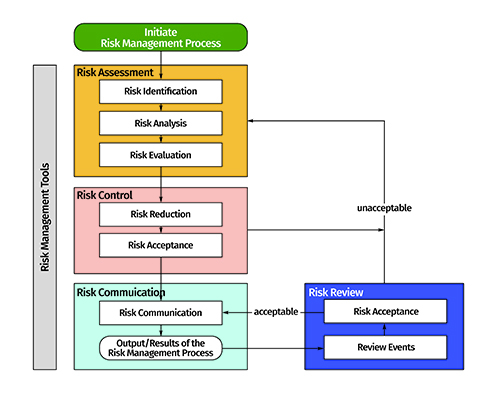
ICH guideline Q9 on quality risk management
ICH guideline Q9 on quality risk management 1 Introduction Risk management principles are effectively utilized in many areas of business and government including finance insurance occupational safety public health pharmacovigilance and by agencies regulating these industries |
|
Final Concept Paper ICH Q9(R1) - Quality Risk Management
ICH Q9(R1) - Quality Risk Management Endorsed by the Management Committee on 13 November 2020 Type of Harmonisation Action Proposed The following harmonization actions are proposed: Limited and specific adjustments would be made to specific chapters and annexes of the current ICH Q9 Guideline on Quality Risk Management (QRM) |
|
ICH guideline Q9 on quality risk management - European Medicines
These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the Page 4 ICH guideline Q9 |
|
Quality Risk Management ICH Q9
prepared by some members of the ICH Q9 EWG for example only; not an official policy/guidance July 2006, slide 1 ICH Q9 QUALITY RISK MANAGEMENT |
|
Final Concept Paper ICH Q9(R1) - Quality Risk Management
ICH Q9(R1) - Quality Risk Management Endorsed by the Management Committee on 13 November 2020 Type of Harmonisation Action Proposed |
|
ICH Q9 - AFMPS
L'objet du présent document est de proposer une approche systématique de la gestion du risque qualité Il sert de base ou de document ressource |
|
Quality Risk Management ICH Q9 - PMDA
http://www sverdrup com/safety/fta pdf ICH Q9 Annex I: Methods Tools prepared by some members of the ICH Q9 EWG for example only; not an official policy/ |
|
Annex 2 - WHO World Health Organization
Reproduced from reference 5: ICH Q9: Quality Risk Management GuidanceComplianceRegulatoryInformation/Guidances/UCM070336 pdf ) 13 Stamatis DH |
|
Formation en Gestion de risques–ICHQ9, FMEA - PBE Expert Inc
2 avr 2018 · Après un rappel des exigences réglementaires (ICH Q9, BP, ISPE): 3 L' approche Risk Management sera étayée et pratiquée à travers plusieurs |
|
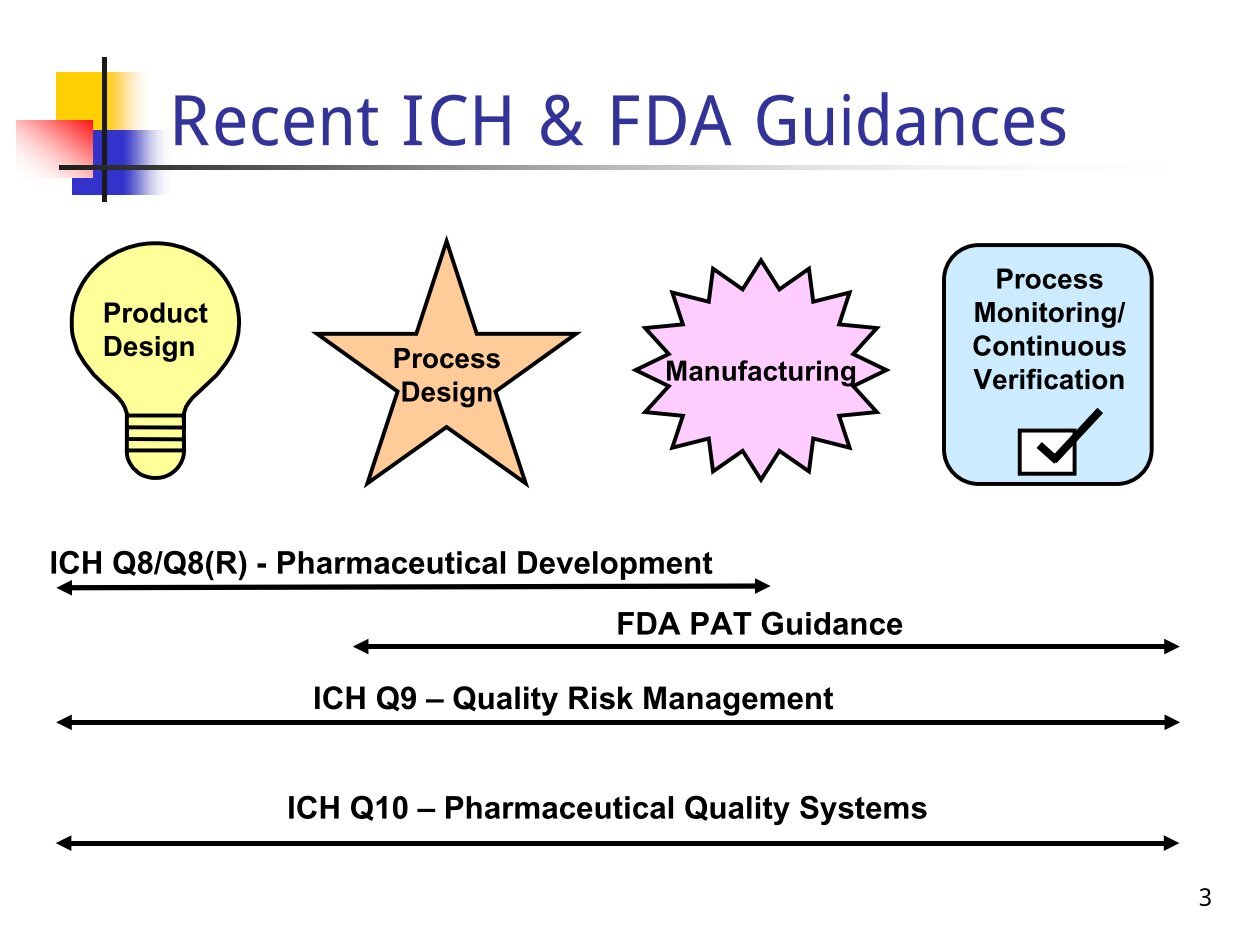
ICH Q8 - Académie Nationale de Pharmacie
2 mar 2011 · De ce fait, les notes explicatives IQH Q8, Q9 et Q10 virent le jour ICH Quality Vision “Develop a harmonized pharmaceutical quality system |
|
Quality Risk Management Principles and Industry Case Studies
ICH Q9 - Quality Risk Management provides an excellent high-level framework for the use of risk management in pharmaceutical product development and |
































![ICH Q9 (eng) - [PDF Document] ICH Q9 (eng) - [PDF Document]](https://le-cdn.website-editor.net/2711a6543f784acca0a8a96da14bfa2d/dms3rep/multi/opt/Picture%2B1-640w.png)



![PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a](https://img.yumpu.com/22459905/1/1250x938/pharmaceutical-development-ich-q8-q8r.jpg)



![PDF] PQLI Application of Science- and Risk-based Approaches (ICH PDF] PQLI Application of Science- and Risk-based Approaches (ICH](https://demo.fdocuments.in/img/378x509/reader024/reader/2021010522/5475e142b4af9fa90a8b5ccf/r-1.jpg)
![Quality Risk Management Tools - [PDF Document] Quality Risk Management Tools - [PDF Document]](https://image.slideserve.com/1278583/ich-q9-quality-risk-management-n.jpg)



![ICH Q9 Quality Risk Management - [PDF Document] ICH Q9 Quality Risk Management - [PDF Document]](https://images.slideplayer.com/23/6587849/slides/slide_3.jpg)













![PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a PDF] Analysis and critical review of ICH Q8 Q9 and Q10 from a](https://images.slideplayer.com/25/8147231/slides/slide_2.jpg)

![ICH Q9 (eng) - [PDF Document] ICH Q9 (eng) - [PDF Document]](https://www.ivtnetwork.com/sites/default/files/images/ICHQ-9%20EWG%20QRM.png)






