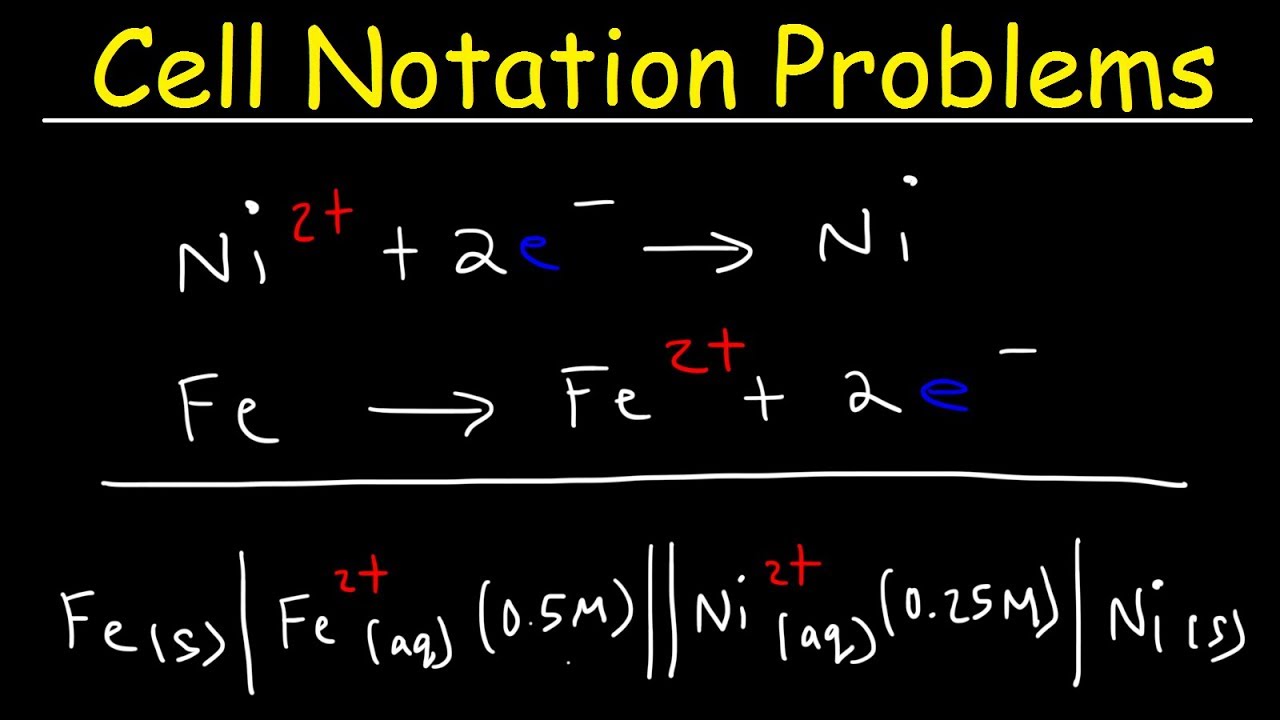
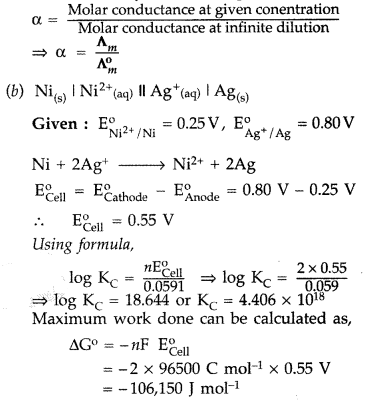
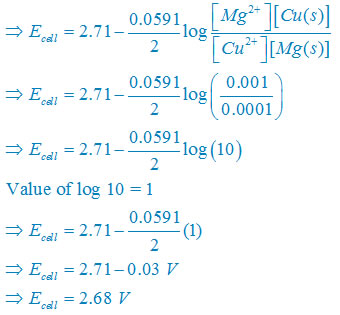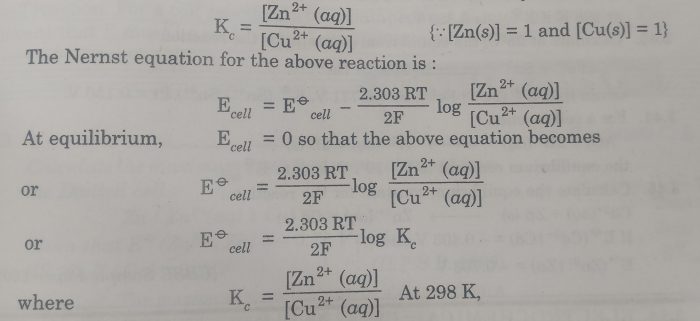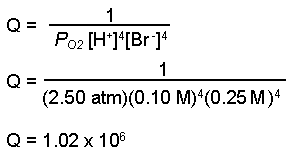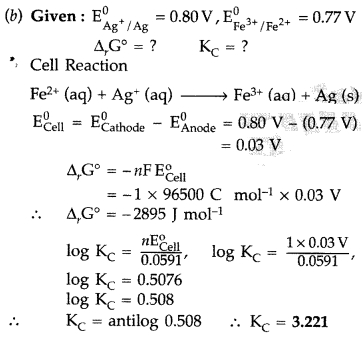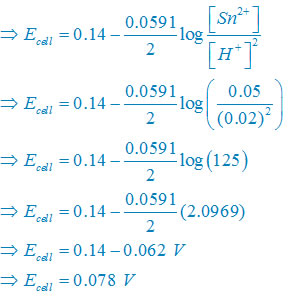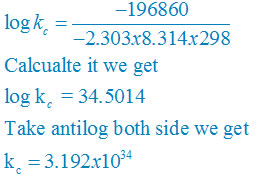calculate the cell potential of the following cell at 25°c
How is cell potential measured?
Cell potential is measured in Volts (=J/C).
This can be measured with the use of a voltmeter.At 25∘C temperature the cell potential of a given electrochemical cell is 1.92 V.
Mg(s)∣∣Mg2+(Aq)xM∣∣∣∣Fe2+(aq)0.01M∣∣Fe(s)
How do you calculate the cell potential of a cell?
The formula for finding cell potential energy is E cell = E cathode - E anode, which is the voltage.
The cell potential energy is positive during a spontaneous reaction.
The formula for finding the electrochemical energy is delta G = -n*F*E.
Delta G must be negative for a reaction to be spontaneous.
|
Test4 ch19 Electrochemistry Practice-answers-Marked
p4 Nonstandard Concentrations and Cell Potential p11. Cell Potentials +0.889 V c. +2.431 V. 25. What is E? for the following balanced reaction? |
|
Untitled
3) A galvanic cell is set up using the following half-reactions: 17) Calculate the volume of H? gas at 25°C and 1.00 atm that will collect at the ... |
|
CHEM 122 Spring 2006
Determine standard cell potentials for oxidation-reduction reactions for the following reaction that takes place in an electrochemical cell at 25°C. |
|
CHEM1612 2014-N-12 November 2014 • In the electrolytic
Nov 12 2014 What is the electrochemical potential of the following cell at 25 ... Calculate the equilibrium constant of the reaction at 25 °C. |
|
Chem 1120 Test 3 Sample Questions
Calculate the cell potential of the following voltaic cell at 25°C. Mg |
|
Untitled
calculate the cell potential E |
|
Untitled
Nov 6 2017 2) The standard cell potential is 1.46 V for a voltaic cell based on the ... to calculate E cell for the voltaic cell based on the following ... |
|
Chapter 18: Electrochemistry
Once the half-cell potentials are determined we can calculate E°cell. E cell e.g. |
|
19 Applications of Standard Electrode Potentials
Ex. 19-1 Calculate the thermodynamic potential of the following cell and the free ?G = -nFEcell = -2 × 96485 C × 0.412 V = -79500J (18.99 Kcal). |
|
General Chemistry II Exam 2
11 mar 2008 · following reaction: NH4+ NH3 +H+by the equation: AGE (55)-11-25) The following standard reduction potentials apply: c) Negative ions pass through the salt bridge from the silver half-cell to the copper half-cell |
|
CH21Apdf
Diagram the following electrolytic cell, indicating the anode, the cathode, the 25 Calculate the cell potential of each of the electrochemical cells at 25°C |
|
Chem 338 Homework Set solutions November 14, 2001 From
14 nov 2001 · 9 17) State what you would expect to happen to the cell potential if the following changes were made to the cells of 6 5: In each case, determine |
|
Problem Set 7 Answers - Chemistry 2300
For the cell at 25 ºC: Zn(s) Zn2+ (4 0 x 10–3 mol L–1, aq) Cd2+ (0 20 mol L–1, aq) Cd(s) (a)Write the cell reaction (b)Calculate the cell voltage (c) Calculate |
|
CHEM 103—Spring 2006 Exam 2 15 May 2006 Name - Cal State LA
15 mai 2006 · _B__ 7 Use the data given to calculate the value of G°rxn at 25°C for the reaction given below In the anode compartment of an electrochemical cell, the electrode is being ______, and Use this list of half-reactions to answer the following question(s) OBJ: 19 7 Effect of Concentration on Cell Potential |
|
1) At 2257 K and 100 atm total pressure, water is 177 per cent
2) Dinitrogen tetroxide is 18 46 per cent dissociated at 25°C and 1 00 bar in the 7) Write the cell reaction and electrode half-reactions and calculate the standard emf 8) Devise cells in which the following are the reactions and calculate the |
|
Concept Check 19 - Cengage
Would the calculated voltage for the cell in part b be different than if you were using the values presented in Table 20 1? Do the calculations to justify your answer you would observe over a several day period if you performed the following |
|
Chapter 5
5 1 The experience curve shown in Fig 5 1 is based on the following equation: C t ( )= C 0( ) N t( ) 5 2 For the simple equivalent circuit of a 0 017 m2 photovoltaic cell shown below, The load current and output power when the output voltage is V = 0 55 V c 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 C u rre |
|
Ft not%\ - International Atomic Energy Agency
calculate the following formation constants, at zero ionic strength: lg (33(V)/B3(VI )) = 111 ± 2 Potential diagrams of neptunium and plutonium Bibliography VTII 0,25 0 11 4+ Y 2 C 0 3 0,80 F e 2 (0H) 24+ 0,75 (0,82) P b 4 (0H) 44+ 0,02 de plus 2) Cell experiments [8] made as titrations: an initial solution of known |
|
RSC_IB_C1IB00128K 19 - CORE
Leukocytes and tumor cells are heterogeneous in their migration responses to flow, yet most 3D chemotaxis 23–25 In the latter mechanism, autologously secreted each cell, we could obtain the following data for each cell as 3 Cell- averaged calculations reveal that interstitial fluid flow increases the migrational |