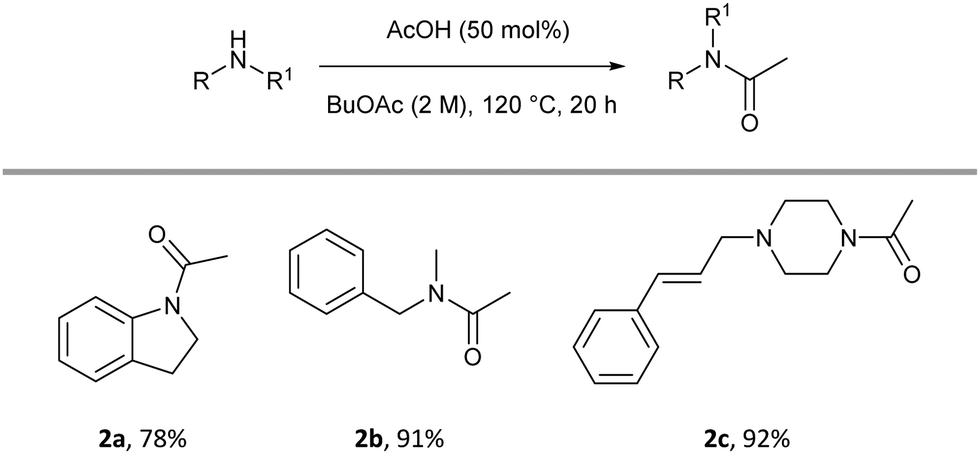
acetylation of amines using acetic anhydride
How is acetylation performed?
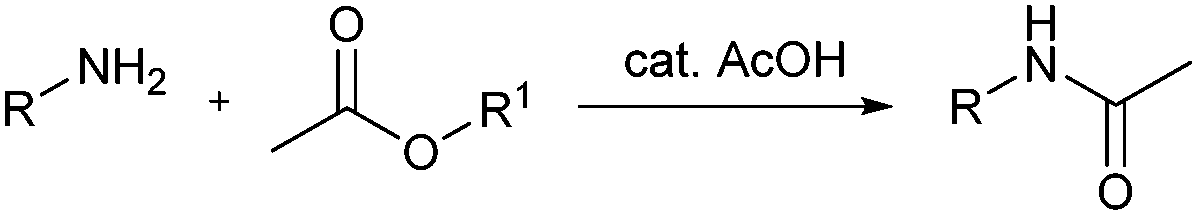
Acetylation reactions are classically performed using excess of acetic anhydride (Ac 2 O) in solvent-free conditions or by eventually working with stoichiometric amounts of Ac 2 O in organic solvents; both methods require the addition of basic or acid catalysts to promote the esterification.
How to acetylate primary amines and amino acids in brine solution?
The present methodology illustrates the efficient acetylation of primary amines and amino acids in brine solution by means of acetyl chloride under weakly basic condition in the presence of sodium acetate and/or triethyl amine followed by trituration with aqueous saturated bicarbonate solution.
Can amines be acylated with acetic anhydride?
Acetylation of amines with acetic anhydride. Amines in the form of amine hydrochlorides are efficiently acylated with anhydrides in an aqueous medium on addition of NaHCO 3 . Both cyclic and acyclic anhydrides react with equal ease with an amine and amines of various stereo-electronic factors react with the same rates with an anhydride.
How acetylation occurs in acetic anhydride?
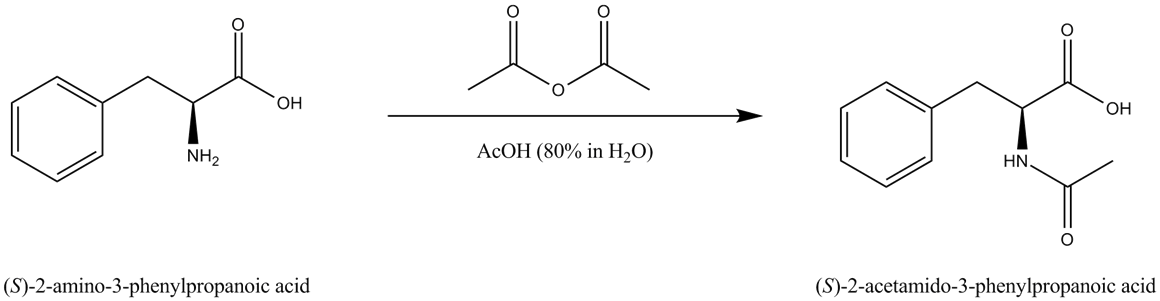
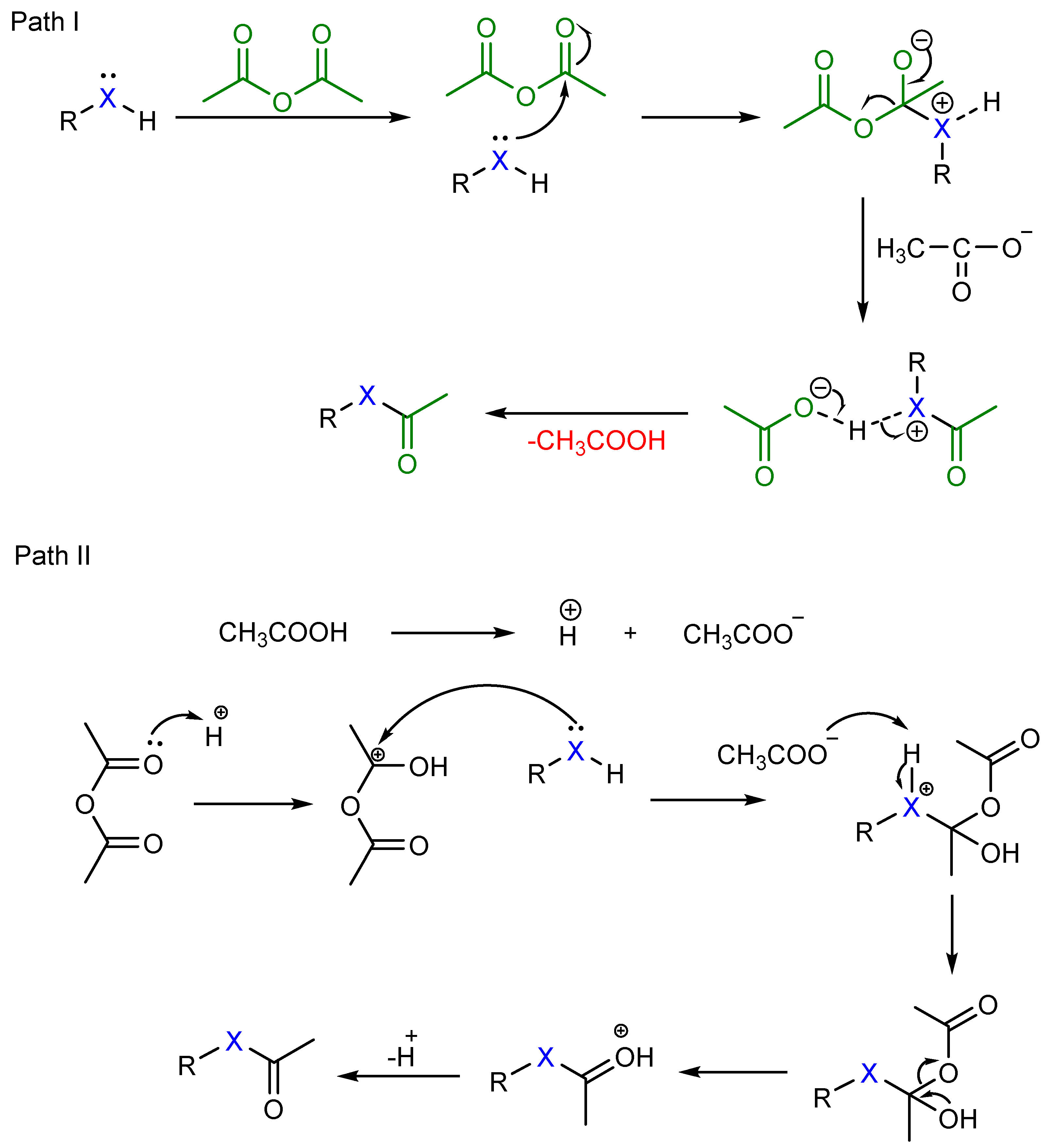
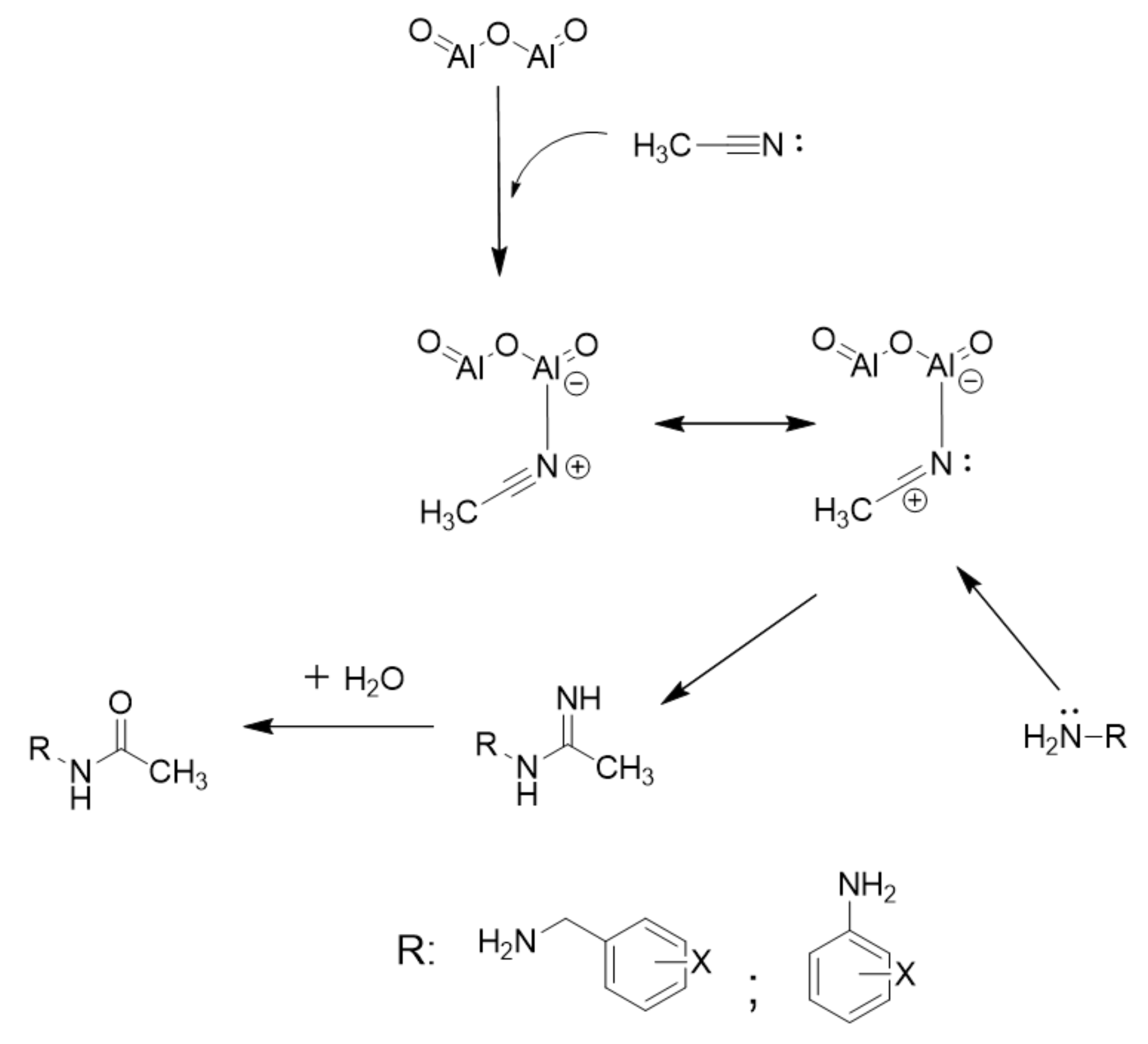
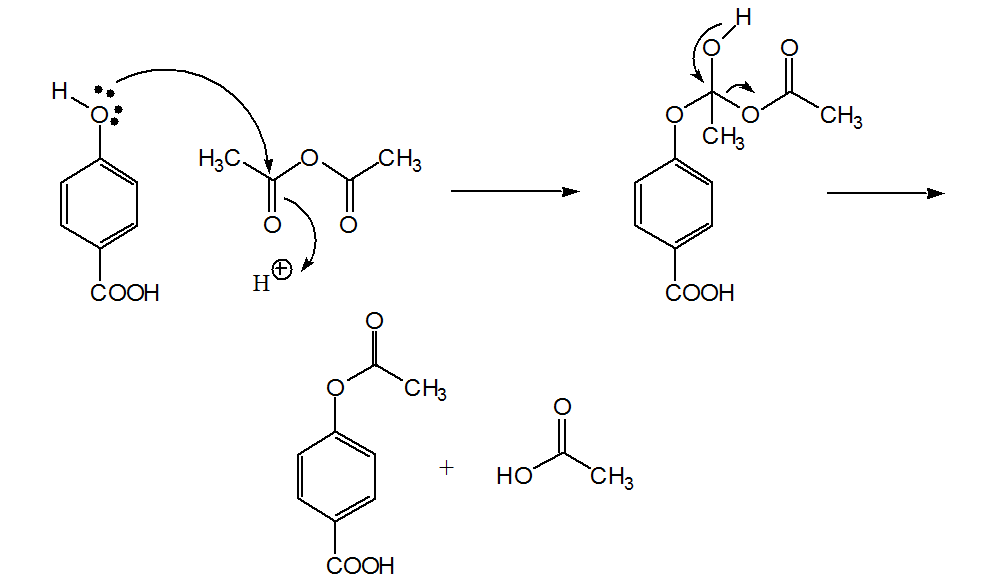
Based on the observed results in Table 1, a suitable mechanism is proposed for the acetylation of alcohols, phenols, and amines ( Scheme 2 ). The lone pair of electrons on oxygen and nitrogen attack the carbonyl group in acetic anhydride to give an adduct which later eliminates acetic acid to give the corresponding ester (Path I).
A Acetylation of Aromatic Primary Amine
5 g (0.038 mol, 1.5 eqv.) of sodium acetate trihydrate was dissolved in 50 ml of brine solution (36 % aq. solution of sodium chloride). To this was added 0.025 mol of the aromatic primary amine (water insoluble amines were taken in ~20 ml acetone). Then 2 ml of acetyl chloride (0.028 mol, 1.1 eqv.) in 3 ml of acetone was added to the mixture drop-w
B Acetylation of Aliphatic Primary Amine
5 g (0.038 mol, 1.5 eqv.) of sodium acetate trihydrate was dissolved in 50 ml brine solution. Then 0.025 mol of the aliphatic primary amine and 3.8 ml (0.028 mol, 1.1 eqv.) triethylamine in 10 ml acetone was added to brine solution and it was followed by drop-wise addition of 1.1 eqv. of acetyl chloride in 3 mL acetone with continuous stirring. Aft
A N-P-Tolylacetamide
FTIR (KBr): 3291, 1685, 1610, 1552 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 7.56 (d, J = 7.7 Hz, 2H), 7.22 (m, Ar -2H & −NH), 2.36 (s, 3H), 2.14 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ164.23, 136.76, 133.77, 128.03 (×2), 122.07 (×2), 24.02, 20.07. link.springer.com
B 4-Acetamidobenzoic Acid
FTIR (KBr): 3306, 1684, 1608, 1592 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 12.42 (s, 1H), 8.32 (d, J = 8.7 Hz, 2H), 7.45 (d, J = 8.7 Hz, 2H), 2.04 (3H, s); 13C NMR (125 MHz, DMSO-d6) δ164.36, 163.23, 136.76, 127.77, 128.03 (×2), 120.07 (×2), 24.21. link.springer.com
C 2-Acetamidobenzoic Acid
FTIR (KBr): 3386, 1701, 1686, 1584 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 12.58 (s, 1H), 8.44 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.40 (m, Ar -1H & –NH ), 7.02 (t, 1H), 2.08 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ165.16, 164.29, 140.16, 130.37, 128.03, 127.58, 120.07, 118.22, 23.31. link.springer.com
D N,N′-(1,4-Phenylene)Diacetamide
FTIR (KBr): 3301, 1708, 1664, 1589 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 7.54 (m, 2H), 7.21 (m, 2H), 2.06 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ164.74(×2), 134.57 (×2), 122.00 (×2), 121.02 (×2), 24.31(×2). link.springer.com
E Sodium 4-Acetamidobenzenesulphonate
FTIR (KBr): 3401, 1705, 1683, 1584 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 7.65 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 2.48 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ166.84, 133.18, 127.00 (×3), 121.79 (×2), 23.31. link.springer.com
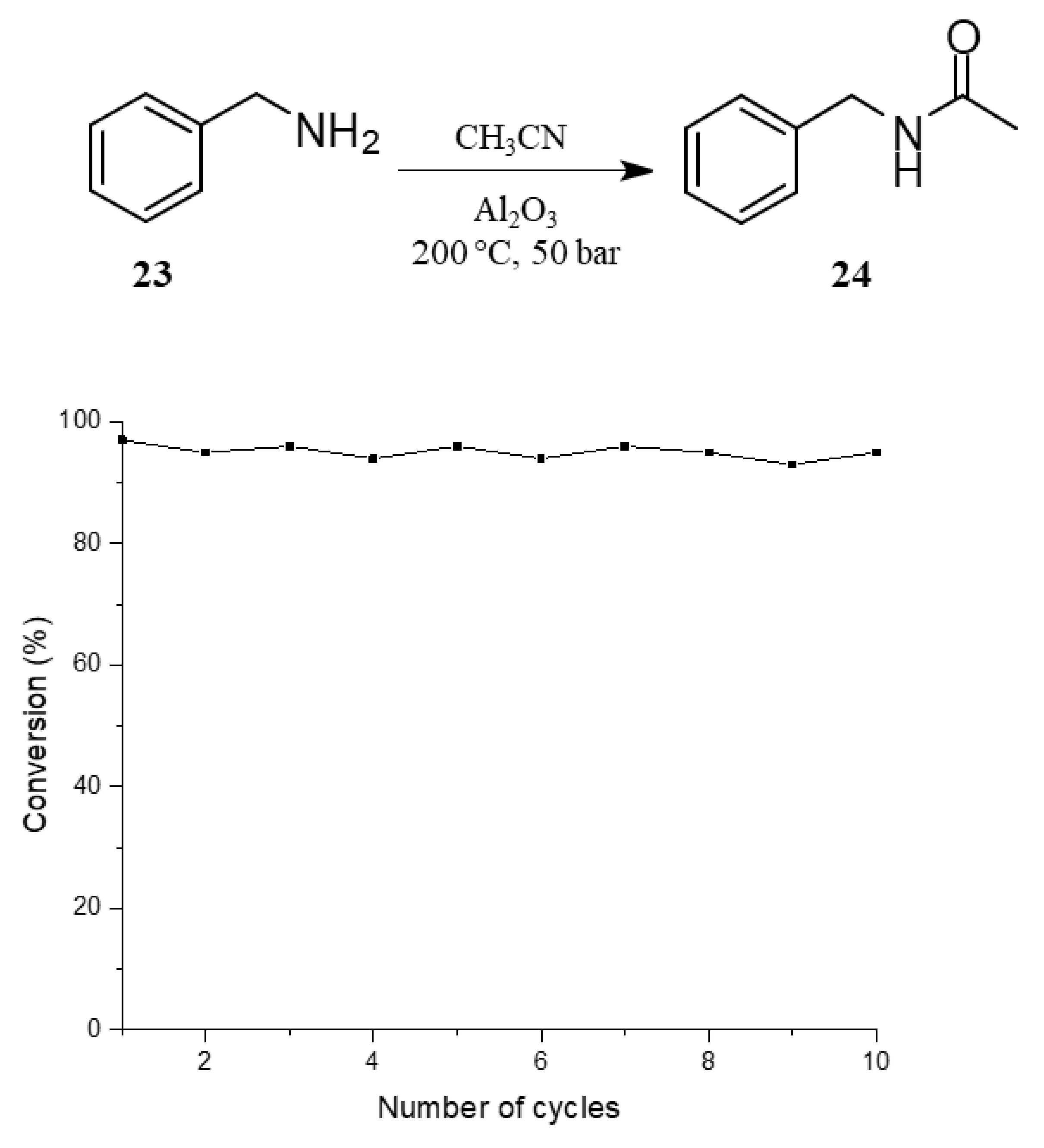
F N-Benzylacetamide
FTIR (KBr): 3297, 1648, 1555 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 7.45–7.23 (m, 5H), 3.84 (s, 2H), 2.04 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ167.54, 137.18, 128.60 (×2), 126.75 (×2), 124.39, 52.34, 24.21. link.springer.com
G 2-Acetamido-3-(1H-Indol-3-Yl)Propanoic Acid
FTIR (KBr): 3358, 3341, 1721, 1712, 1629, 1552 cm− 1; 1H NMR (500 MHz, DMSO-d6) δ 12.57 (br, 1H), 10.83 (s, 1H), 8.14 (d, J = 7.5 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.13 (s, 1H), 7.06 (t, 1H), 6.97 (t, 1H), 4.45 (q, 1H), 3.15, 2.98 (m, 2H, Ar-CH2, diastereotopic), 2.11 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ169.23, 164.19, 1

Acetylation of amine amine react with acetyl chloride or acetic anhydride

Acetylation Reaction Mechanism-Organic Chemistry

Acylation using an anhydride
|
A Novel SN1 Displacement: The Reaction of Tertiary Amines with
In the course of studying the acetylation of amines (1) we have examined the reaction of several tertiary amines with acetic anhydride. It was found that |
|
Mild and eco-friendly chemoselective acylation of amines in
8 The same has been achieved using amine hydrochloric acid |
|
Metal Acetate/Metal Oxide in Acetic Acid: An Efficient Reagent for
8 juin 2010 This alternative method for N-acetylation of amines avoids the use of conventional acetylating agents (acetyl chloride or acetic anhydride) and ... |
|
Quantitative Acetylation of Amines by Means of Acetyl Chloride and
tially in modifying theusual acetic anhydride- pyridine acetylation mixture values of amines using the Smith-Bryant reagent. 3. A new acetylating ... |
|
Efficient acetylation of primary amines and amino acids in
put forward for the acylation of amines using acetic anhydride such as |
|
Note A green approach to chemoselective N- acetylation of amines
The reaction procedure requires no other solvent and is rapid with good to excellent yields. Keywords: Microwave irradiation |
|
NN-DIACETYLAMINES AS BY-PRODUCTS FROM THE
Acetylation of five alicyclic amines and isopropylamine with a large excess of acetic anhydride for 1 hour under reflux led to formation of appreciable |
|
ACETIC ANHYDRIDE CAS N°: 108-24-7
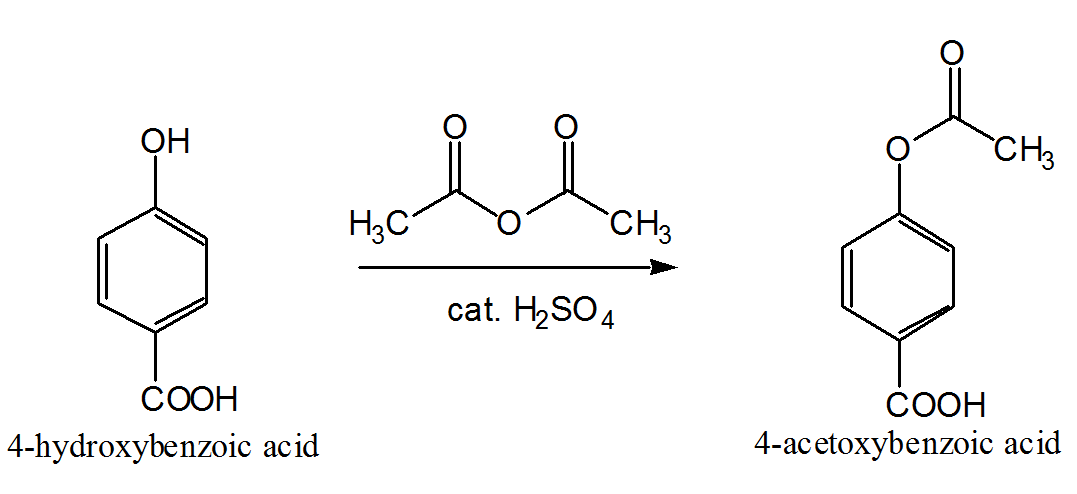
Reactions of acetic anhydride with hydroxyl groups yield the corresponding acetate ester with coproduction of acetic acid. Acetylation of amines produce |
|
Acetic acid as a catalyst for the N-acylation of amines using esters
21 nov. 2016 We report a cheap and simple method for the acetylation of a variety of amines using catalytic acetic acid and either ethyl acetate. |
|
Catalytic Acetylation of Amines with Ethyl Acetate
The reaction is usually achieved through acetylation with acetic anhydride /l/ or under basic conditions /2 |
|
Facile and Efficient Acetylation of Primary Alcohols and Phenols with
3 déc. 2013 tertiary amine bases such as either triethylamine or pyridine [12]; Lewis ... acetylation of alcohols using acetic anhydride |
|
Efficient acetylation of primary amines and amino acids in
put forward for the acylation of amines using acetic anhydride such as |
|
A Novel SN1 Displacement: The Reaction of Tertiary Amines with
In the course of studying the acetylation of amines (1) we have examined the reaction of several tertiary amines with acetic anhydride. |
|
Metal Acetate/Metal Oxide in Acetic Acid: An Efficient Reagent for
Keywords: amines N-acetylation; chemoselectivity |
|
Simple and Efficient Method for Acetylation of Alcohols Phenols
Abstract: Solvent-free acetylation of alcohols phenols |
|
Mild and eco-friendly chemoselective acylation of amines in
9 We thought to add sodium bicarbonate to an aqueous solution of amine hydrochloride which will liberate free amine and react with acetic anhydride and convert |
|
An Improved and Efficient N-acetylation of Amines Using Choline
An optimization on amine:acetic acid anhydride (AAA) ratio was performed on anthranilic acid in a DES made of choline chloride (ChCl) and malonic acid (Table 1) |
|
M(acac)n Covalently Anchored onto Amine Functionalized Silica
7 nov. 2012 transformations are usually performed with acetic anhydride and/or acetyl ... and efficient method for the acetylation of amines phenols. |
|
Note A green approach to chemoselective N- acetylation of amines
amines using catalytic amount of zinc acetate in acetic acid under microwave irradiation. carried out with acetic anhydride or acetyl chloride in. |
|
Efficient acetylation of primary amines and amino acids - Indian
put forward for the acylation of amines using acetic anhydride, such as, acetic anhydride in acetic acid,3 acetic anhydride in aqueous sodium bicarbonate4 solu - |
|
Acetylation of amines using catalytic amount of zinc acetate - NOPR
Instead of using such reagents, acetic anhydride (or acetyl chloride) is still regarded as the key N-acetylating agent, both in commercial as well as non- commercial |
|
An Efficient Method for Acylation Reactions - ScienceDirectcom
Abstract: Cu(OTf)2 was found to be an efficient catalyst in the acylation reaction of alcohols, phenols, amines and thiols with acetic anhydride in CH2C12 or |






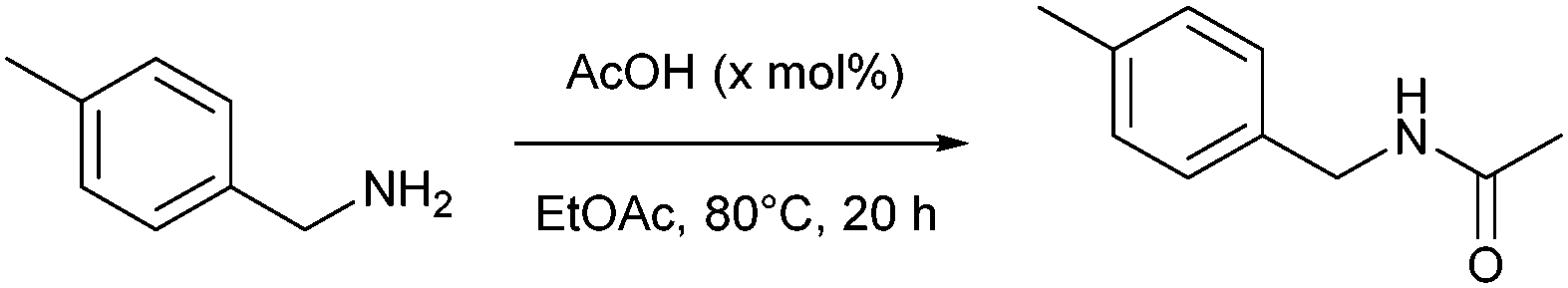









![PDF] Derivatization with acetic anhydride: applications to the PDF] Derivatization with acetic anhydride: applications to the](https://patentimages.storage.googleapis.com/2b/4a/01/24b7f1273ccf7d/imgb0001.png)












![PDF] Facile and Efficient Acetylation of Primary Alcohols and PDF] Facile and Efficient Acetylation of Primary Alcohols and](https://magritek.com/wp-content/uploads/2019/10/Fig3_Reaction_setup.png)

