acid catalyzed hydrolysis of a fat
What is a hydrolysis reaction?
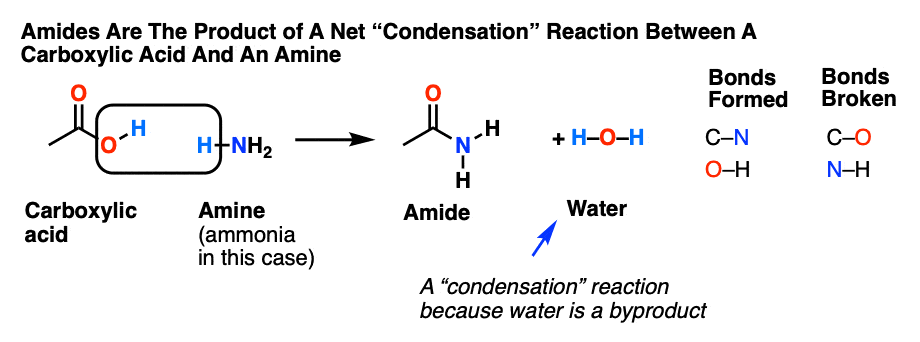
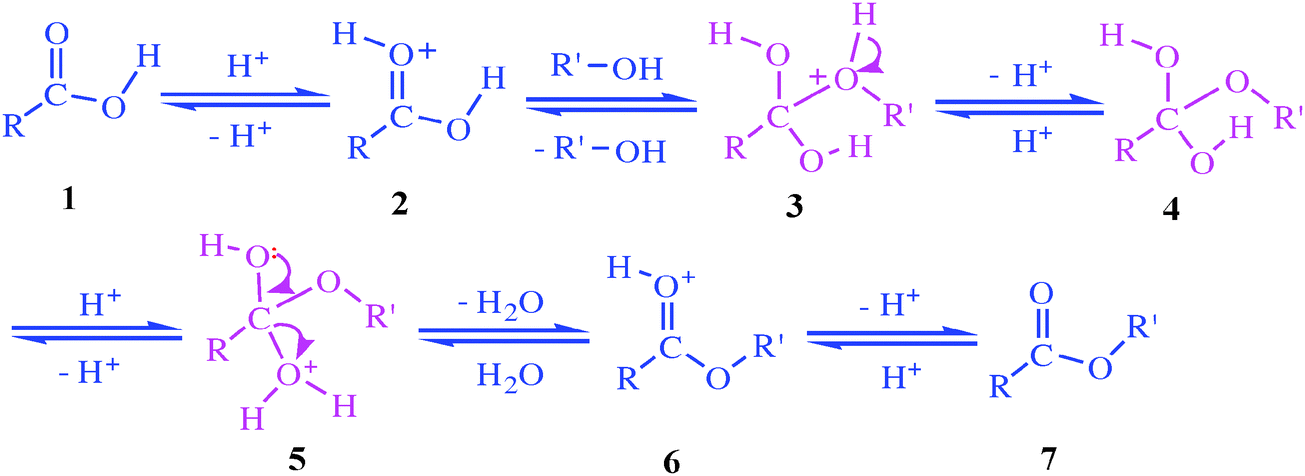
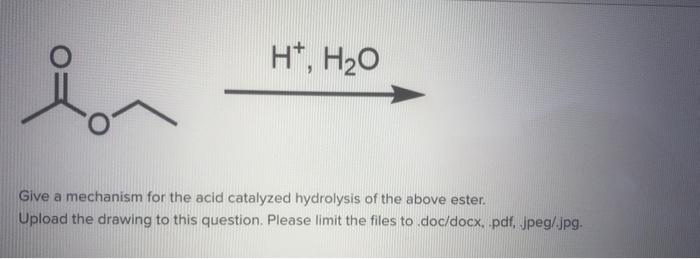
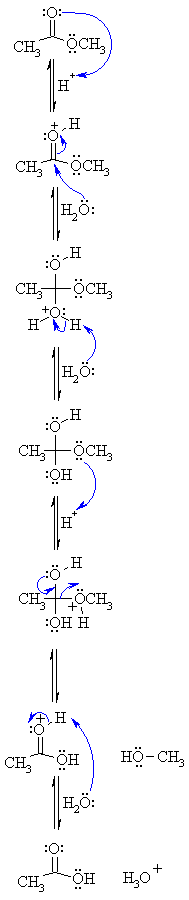
Hydrolysis is “splitting with water.” Hydrolysis is the most important reaction of esters. The hydrolysis of esters is catalyzed by either an acid or a base. During acid catalysed hydrolysis the ester is heated with water containing a small amount of a strong-acid catalyst such as sulfuric acid.
Why are fats and oils hydrolyzed?
Fats and oils can participate in a variety of chemical reactions—for example, because triglycerides are esters, they can be hydrolyzed in the presence of an acid, a base, or specific enzymes known as lipases. The hydrolysis of fats and oils in the presence of a base is used to make soap and is called saponification.
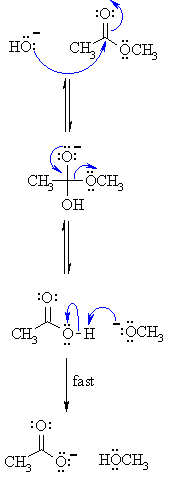
What is base-catalyzed ester hydrolysis?
The base-catalyzed ester hydrolysis is also known as saponification because it is used in the production of soaps from fats. Remember, soap is a salt of a fatty acid and can be formed when a fat (an ester derived from a glycerol and three molecules of fatty acid) is hydrolyzed by base catalysis:
What is alkaline hydrolysis of oils & fats?
Alternatively to the acid catalysts, alkaline hydrolysis of oils and fats has also been carried out, in particular with residual TAGs . However, in this case, saponification reaction occurs (i.e. soap-making) and the product is the corresponding alkaline salt of the fatty acids .
Effect of Process Parameters and Statistical Analysis
D-optimal design optimization was employed to study the percentage of free fatty acid (FFA%) by ethanolic KOH concentration hydrolysis of J. curcas seed oil. Experimental results of FFA% for the ethanolic KOH concentration effects to J. curcas seed oil hydrolysis are given in Table 1. The results show the hydrolysis performance of the ethanolic KOH
GC-FID Analysis of Fatty Acids Composition
Response surface methodology (RSM) was employed to study the composition of FFA by ethanolic KOH concentration of J. curcas seed oil hydrolysis through FAMEs analysis before and after the hydrolysis. The analyses made by GC-FID had a positive identification of FAs. Experimental results of the percentage of the composition of FAs for ethanolic KOH r
FTIR Analysis of Fatty Acids
In order to prove the J. curcas seed oil hydrolysis, FTIR spectroscopy supported the FFA% by showing the main peaks and their functional groups of the J. curcas seed oil. The comparison between J. curcas seed oil (a), hydrolysis at 1.00M (b) and at 1.75M of ethanolic KOH concentration (c), FTIR spectra is shown in Figure 6. The main peaks and their
HPLC Analysis of Fatty Acids
The results by using higher performance liquid chromatography (HPLC) show the hydrolysis performances of the ethanolic KOH concentration effects on the hydrolysis reaction when submitted to different concentrations of the ethanolic KOH (1.0, 1.5 and 1.75M). The study of variation yield of the hydrolysis J. curcas seed oil has been showed in Figures
Experimental
Procedure of J. curcasSeed Oil Hydrolysis FFA was obtained by the hydrolysis of J. curcas seed oil, as carried out by [19]. Table 6 shows different ethanolic KOH concentration, different reaction temperature and different reaction time using RSM (D-optimal design). Factors such as ethanolic KOH concentration (M, X1 ), temperature (°C, X2 ) and time (h, X3 ) were performed under the same experimental conditions. In a typical experiment, J. curcas seed oil 50 g was mixed in the reactor with 300 mL of saponifying solution comprising of
Determination of The Ffa%
The FFA% of the hydrolysis of J. curcas seed oil was determined according to [20]. Approximately 50 mL of isopropanol was placed into the flask, and about 0.5 mL phenolphthalein was added and was neutralised by addition of sodium hydroxide (NaOH, 0.02N) until a permanent pink colour was obtained. The neutralised isopropanol was added to the 5 g of
Gas Chromatography Method Analysis of Fatty Acids Composition
Gas chromatography method (GC) analysis was performed on Shimadzu equipped with flame ionization detector and capillary column (30 m × 0.25 mm × 0.25 mm film). The parameters of GC have been carried out according to [21]. bmcchem.biomedcentral.com
Fourier Transforms Infrared Spectroscopy Analysis of Fatty Acids
Fourier transforms infrared spectroscopy (FTIR) has been carried out according to [21]. FTIR of the products was recorded on a Perkin Elmer Spectrum GX spectrophotometer in the range 400-4000 cm-1. FTIR was used to measure functional groups of FA. A very thin film of FA was covered on NaCl cells (25 mmi.d × 4 mm thickness) and was used for analysis
High Performance Liquid Chromatography Method Analysis of Fatty Acids
High performance liquid chromatography (HPLC) was performed on waters model 1515 equipped with refractive index detector and Spherisorb C18 column (250 mm × 4.8 mm × 3 mm) was used for analysis the TAG, DAG, MAG and FFA. The parameters of HPLC have been carried out according to [21]. The samples were dissolved in 10 mL of the mixture acetone: aceto
Experimental Design (D-Optimal) and Statistical Analysis
A three-factor D-optimal design was employed to study the responses of FFA% [Y in % by wt, see Eq. (2)]. An initial screening step was carried out to select the major response factors and their values. The independent variables were X1, X2 and X3 representing the concentration of ethanolic KOH (M), reaction temperature (°C), and reaction time (h),

Acid-catalyzed ester hydrolysis Organic chemistry Khan Academy

Ester Hydrolysis Reaction Mechanism

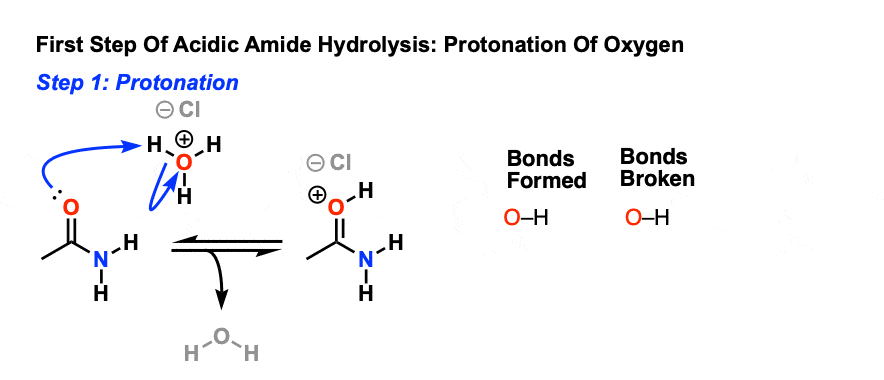
Acid and base-catalyzed hydrolysis of amides Organic chemistry Khan Academy
|
Investigating the Hydrolysis of Fats and Oils
Hydrolysis can break down a fat or oil and release the triglycerol and fatty acids. The acids can be separated and identified and this information can be used |
|
A Robust Two-Step Process for the Efficient Conversion of Acidic
7 nov. 2018 hydrolysis was adopted first then the hydrolyzed free fatty acid |
|
Interesterification of Milk Fat with Oleic Acid Catalyzed by
Fatty acid standards triglyceride standards |
|
Mechanistic Modeling of Hydrolysis and Esterification for Biofuel
12 oct. 2011 acid ester hydrolysis and fatty acid (FA) esterification when each ... hydrolysis of fatty acid alkyl esters includes catalysis by H+ by. |
|
Hydrolysis Characteristics of Bovine Milk Fat and Monoacid
15 avr. 1997 and kids and the resulting profiles for free fatty acids ... goat and kid pregastric enzyme catalyzed hydrolysis of anhydrous milk fat were ... |
|
Total Fatty Acid Analysis of Human Blood Samples in One Minute by
27 déc. 2018 (B) Negative ion mode FTMS spectrum of a total FA extract of human plasma using 18O-enriched H2O for acid-catalyzed hydrolysis. (C–F) Selected m ... |
|
Liquid lipaseâ•catalyzed hydrolysis of gac oil for fatty acid
3 oct. 2018 Liquid Lipase-Catalyzed Hydrolysis of Gac Oil for Fatty Acid Production: Optimization Using Response Surface Methodology. Chia-Hung Su. |
|
Application of Lipolytic Enzymes to Flavor Development in Dairy
Hydrolysis of milk fat catalyzed by en- lipolysis catalyzed by indigenous milk lipase ... total free fatty acid contents of various dairy. |
|
Serine-Lysine Catalytic Triad of Fatty Acid Amide Hydrolase
Additional assays conducted with 0.01 and 0.25% Triton X-100-afforded kcat and Km values for. FAAH-catalyzed oleamide and OME hydrolysis equivalent to the |
|
21.7 HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES
Despite its association with fatty-acid esters the Acid-Catalyzed Ester Hydrolysis Because esterification of an acid with an alcohol is a. |
|
Hydrolysis of fats and oils by moist oat caryopses - CORE
Lipase catalyzes the hydrolysis of glycerol esters to yield free fatty acids Various plant lipases have been isolated and characterized It has been known for a |
|
Investigating the Hydrolysis of Fats and Oils
An enzyme called lipase catalyses the hydrolysis of the fats and oils When the hydrolysis occurs the fatty acids will be released and the acidity of the reaction mixture will rise An alkali can be added to the reaction mixture to neutralise the fatty acids |
|
Chapter 4 ENZYMATIC MODIFICATION OF OILS AND FATS
According to the results, polyunsaturated fatty acids are the most resistant to lipase-catalyzed hydrolysis Figure 4-1-7: Determination of relative fatty acid specificity |
|
Quantitative release of fatty acids from lipids by a simple hydrolysis
at 100°C or 4 hr at 70°C After hydrolysis, free fatty acids (FFA) are recovered in tained for methyl esters using methanolysis catalyzed by acid, alkali, or BFS |
|
Non-catalytic steam hydrolysis of fats and oils by - ScholarWorks
The hydrolysis of fat and oil triglycerides to free fatty acids and glycerol requires conditions which promote triglyceride and water miscibility The reaction is not |
|
KINETIC MODEL FOR TRIGLYCERIDE HYDROLYSIS - Neliti
ester bonds of triglyceride are not evenly catalyzed by lipase Although some researchers reported the produced fatty acid inhibited the hydrolysis [20,72,73] |
|
49 Triglycerides(Triacylglycerols) One class of esters is particularly
Triglycerides (fats) can be hydrolyzed to produce glycerol and 3 fatty acids in the presence of acid and heat or with a suitable lipase enzyme under biological |



















![PDF] Activation Energies for an Enzyme-Catalyzed and Acid PDF] Activation Energies for an Enzyme-Catalyzed and Acid](https://image.slidesharecdn.com/esterhydrolysis-130213014134-phpapp02/95/acid-base-catalysed-ester-hydrolysis-9-638.jpg?cb\u003d1360719744)