acid hydrolysis in inorganic chemistry
|
Ligand Displacement Reactions in Octahedral Complexes
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is formed due to the replacement of a ligand by water molecule It has been observed that NH 3 ammines like ethylene diamine or its derivatives coordinated to Co 3+ |
|
MSc Semister I Paper I Inorganic Chemistry
Acid hydrolysis reactions occur in neutral and acidic solutions (pH |
Does ammonium ion undergo acid hydrolysis?
The ammonium ion is the conjugate acid of the weak base ammonia, NH 3, and so it will undergo acid ionization (or acid hydrolysis ): Salts are ionic compounds composed of cations and anions, either of which may be capable of undergoing an acid or base ionization reaction with water.
What are acid hydrolysis reactions?
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is formed due to the replacement of a ligand by water molecule. It has been observed that NH3, ammines like ethylene diamine or its derivatives coordinated to Co3+ are displaced at a very small rate.
How does H2O2 affect the rate of base hydrolysis reaction?
Which increase the rate of base hydrolysis reaction and form peroxo products. On the other hand if the reaction occurs by an SN1CB mechanism the addition of H2O2 to the reaction mixture should reduce the rate of base hydrolysis reaction compared to OH- because of the reduction in the concentration of OH- ions.
What is the difference between acidic hydrolysis and basic hydrolysis?
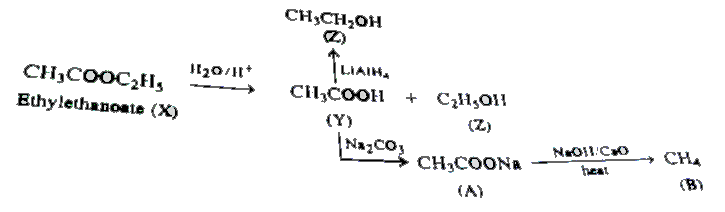
A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol. Basic hydrolysis (saponification) of an ester gives a carboxylate salt and an alcohol. The hydrolysis of an amide produces a carboxylic acid and ammonia or an amine.

Acid hydrolysis and Aquation

Hydrolysis and Dehydration Synthesis Reactions

Hydrolysis and Dehydration Synthesis
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
“A Textbook of Inorganic Chemistry – Volume 1 by Mandeep Dalal” is now ❖ Ligand Displacement Reactions in Octahedral Complexes- Acid Hydrolysis Base ... |
|
M.Sc Semister I Paper I Inorganic Chemistry Department of
Inorganic Chemistry. Department of Chemistry. L.S COLLEGE MUZAFFARPUR. B. R. A. Acid hydrolysis reactions occur in neutral and acidic solutions. (pH<3) while ... |
|
BSc Chemistry
3 and Inorganic Chemistry-I (Stereochemistry Metal-. Ligand Equilibria rate for base hydrolysis over acid hydrolysis is proton abstraction from coordinated. |
|
V. the hydrolysis of organic phosphorus compounds by dilute acid
hydrolysis of phytic acid by acids was required for the estimation of inorganic phosphoric acid in the presence of phytic acid and for the comparison of the. |
|
Nanocellulose prepared by acid hydrolysis of isolated cellulose from
Inorganic and Physical Chemistry Research Division Institut Teknologi Bandung |
|
MECHANISMS OF CLEAVAGE OF GLUCOSE-1-PHOSPHATE
drolyzing it in acid and analyzing the inorganic phosphate formed since mechanism may be due to partial hydrolysis by ordinary acid hydrolysis under the ... |
|
Roxithromycin degradation by acidic hydrolysis and photocatalysis
٢٨/٠٥/٢٠١٤ Complete List of Authors: Kwiecień Anna; Jagiellonian University |
|
Kinetics of the Acid Hydrolysis of Dicyanobis (2 2-bipyridine) iron (II
Inorganic Chemistry. Fig. 2.—Effect of acid on the rate of hydrolysis of Fe(bipy)2-. (CX)2 and Fe(phen)2(CN)2at 50°: · experiments in buffer solutions; O |
|
Acid-catalyzed hydrolysis of acyl phosphates
propyl alcohol-water as the solvent showed the compounds to contain a trace of inorganic phosphate. The compounds were purified for elemental analysis |
|
Cellulose hydrolysis using ionic liquids and inorganic acids under
٢٨/١٠/٢٠٢٠ As a benchmark comparison CNCs were prepared by mineral acid hydrolysis under conventional conditions and the physico- chemical properties ... |
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
A Textbook of Inorganic Chemistry – Volume I Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is. |
|
M.Sc Semister I Paper I Inorganic Chemistry Department of
Inorganic Chemistry. Department of Chemistry. L.S COLLEGE MUZAFFARPUR ligand by H2O molecules are called acid hydrolysis or equation reactions. |
|
BSc Chemistry
Module No. 25: Acid and Base hydrolysis Reactions without metal ligand bond cleavage. Subject. Chemistry. Paper No and Title. 3 and Inorganic Chemistry-I |
|
Reaction Mechanism of Transition Metal Complexes – I
A Textbook of Inorganic Chemistry – Volume I Ligand Displacement Reactions in Octahedral Complexes- Acid Hydrolysis. Base Hydrolysis. |
|
HYDROLYSIS 2016.pdf
mechanisms account for neutral acid and base hydrolysis. Therefore |
|
Acid Hydrolysis of Co(III) amine complexes
The values of rates of acid hydrolysis of some Co(III) complexes at pH= 4 G. L. Miessler and D. A. Tarr ?Inorganic Chemistry? 3rd Ed Pearson/Prentice ... |
|
S.No. Topic Page No. PHYSICAL CHEMISTRY INORGANIC
(d) Strong acid & strong base --------do not hydrolysed------- pH = 7. Hydrolysis of ployvalent anions or cations. For [Na3PO4] = C. Ka1 × Kh3 = Kw. |
|
Roxithromycin degradation by acidic hydrolysis and photocatalysis
28-May-2014 Complete List of Authors: Kwiecie? Anna; Jagiellonian University |
|
M.Sc. (Chemistry) I Semester (w.e.f. 2016 and onwards)
02-Sept-2016 substitution acid hydrolysis |
|
Kinetics of the acid hydrolysis (aquation) - CORE
Part of the Inorganic Chemistry Commons This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and |
|
BSc Chemistry - e-PG Pathshala
Module No 25: Acid and Base hydrolysis, Reactions without metal ligand bond cleavage Subject Chemistry Paper No and Title 3 and Inorganic Chemistry-I |
|
Reaction Mechanism in Transition Metal Complexes
Faculty: Science Department: Chemistry Course: M Sc Semester: II Paper: I, Inorganic Chemistry Unit: 4 Topic: Mechanism of Acid Hydrolysis |
|
HYDROLYSIS
mechanisms account for neutral, acid and base hydrolysis Therefore, the overall The hydrolysis rates of halogenated aliphatic compounds is influenced by |
|
BASE HYDROLYSIS OF COBALT(III) - ACS HIST
of chlorosuccinic acid to form malic acid (Eq 1) Bailar(7) in 1934 discovered what he termed the first "Walden inversion" reaction of inorganic chemistry (Eq 2 ) |
|
Mechanism of the Acid Hydrolysis of Pentammine Cobalt - Nature
Present address: Mobil Chemical International, Ltd , 150 East 42nd Street, New York mechanism for the acid hydrolysis reaction (l) has not, as yet, been Basolo, F , and Pearson, R G , Mechanisms of Inorganic Reactions (J Wiley and |