acid hydrolysis of ester experiment
|
IV SEMMESTER
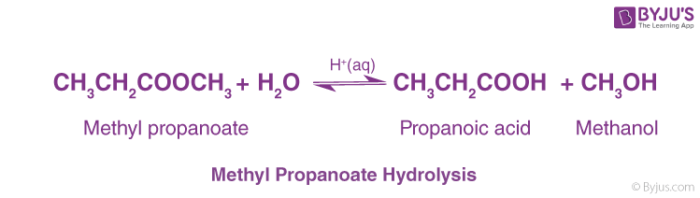
1 KINETICS OF ACID HYDROLYSIS OF AN ESTER AIM: To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as a catalyst PRINCIPLE: The hydrolysis of an ester occurs according to the equation CH3COOC2H5 + H2O CH3COOH + C2H5OH This reaction follows pseudo first order kinetics PROCEDURE: |
How do you synthesis an ester?
The synthesis of an ester can be accomplished in one of several ways. In this lab, esterification occurs when an alcohol and a carboxylic acid are reacted in the presence of an acid catalyst, such as concentrated sulfuric acid. Other synthetic pathways to esters also exist. Acid chlorides react with alcohols to yield an ester and hydrochloric acid.
How is ester hydrolysis reversible?
The hydrolysis of esters is catalyzed by either an acid or a base. Acidic hydrolysis is simply the reverse of esterification. The ester is heated with a large excess of water containing a strong-acid catalyst. Like esterification, the reaction is reversible and does not go to completion.
How does pH affect ester hydrolysis?
kH2O [H2O]. Ester hydrolysis has been shown to be accelerated by both acid and base so the rate is pH dependent as shown below. At high pH, the dependence of log k vs pH increases with a slope of +1 (specific base ‘catalysis’). In general, reaction with OH− is important even at pH values below pH 7.
Equipment
Apparatus 1. Eye protection 2. Glass specimen tubes x4 (note 3) 3. Plastic dropping pipettes, access to adequate supply 4. Beaker, 100 cm3 or 250 cm3(note 3) 5. Test tubes x4 6. Test tube rack 7. Bunsen burner 8. Heat resistant mat 9. Tripod 10. Gauze 11. Crucible tongs Chemicals 1. Glacial (concentrated) ethanoic acid (CORROSIVE), about 2 cm3 2. Propanoic acid (CORROSIVE), about 2 cm3 3. Benzoic acid (HARMFUL), about 0.2 g 4. Concentrated sulfuric acid (CORROSIVE), 5–10 drops (note 7) 5. Access to the following alcohols (about 10 drops of each required) (note 8): 5.1. Methanol (HIGHLY FLAMMABLE, TOXIC) 5.2. Ethanol (HIGHLY FLAMMABLE, HARMFUL if using Industrial Denatured Alcohol, IDA) 5.3. Propan-1-ol (HIGHLY FLAMMABLE, IRRITANT) 5.4. Butan-1-ol (HARMFUL) 6. One or m
Health, Safety and Technical Notes
Read our standard health and safety guidance.Wear goggles throughout.The essential requirements for these tubes are: neutral borosilicate glass a wide flat base, so that they are stable when stood in a beaker. If not available, small test tubes could be used instead
Procedure
Add 10 drops of ethanoic acid (or propanoic acid) to the sulfuric acid in the specimen tube.Add 10 drops of ethanol (or other alcohol) to the mixture.Put about 10 cm3 of water into the 100 cm3beaker. Carefully lower the tube into the beaker so that it stands upright.Heat the beaker gently on a tripod and gauze until the water begins to boil, then stop heating. edu.rsc.org
Teaching Notes
This method is an updated version of the traditional test tube scale approach to ester preparation, which minimises the risks involved in handling the reagents involved. For further information about this method of ester preparation, consult CLEAPSS Guidance Leaflet PS67-07 ‘Making esters’. This method is only suitable for preparing small samples f

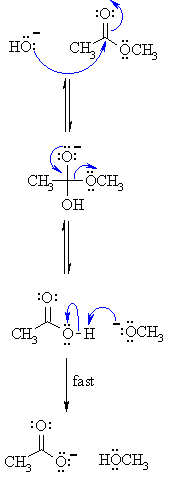
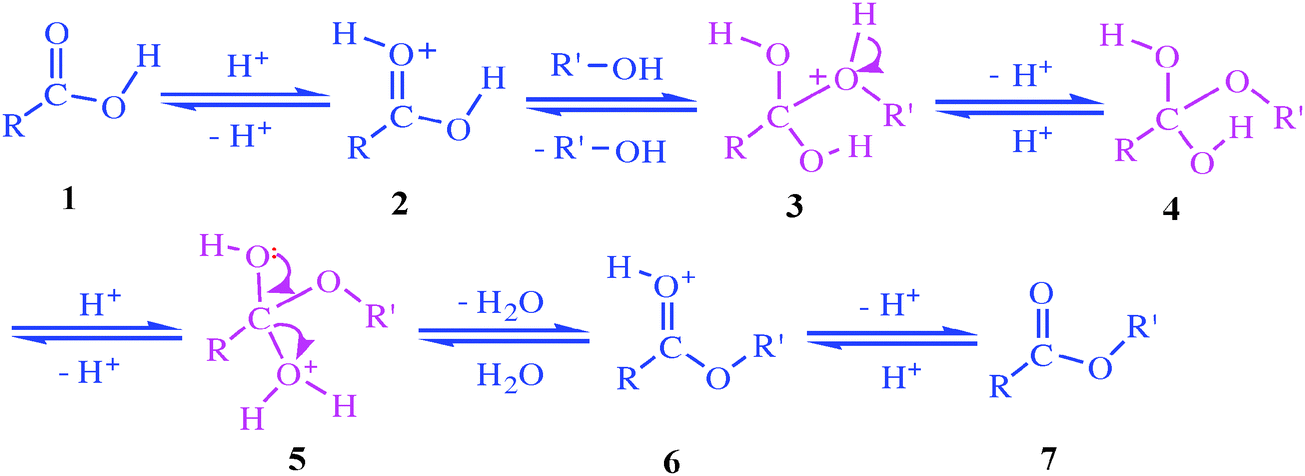
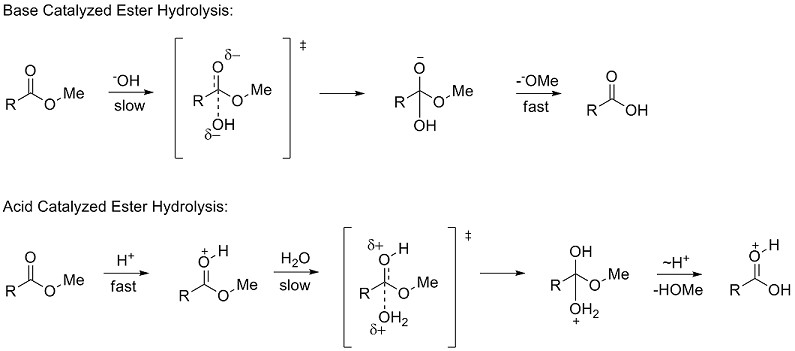
Ester Hydrolysis Reaction Mechanism

Acid-catalyzed ester hydrolysis Organic chemistry Khan Academy

Acid catalysed ester hydrolysis (Chemical kinetics)
|
IV SEMMESTER
EXPERIMENT. Page No. 1. Kinetics of Acid hydrolysis of an ester To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as a. |
|
Page 1 of 12 CHEM 100L Lab 7: Ester Hydrolysis Purpose: In the
Purpose: In the first part of this virtual lab experiment you will use Beyond Labz to perform the acidic hydrolysis of methyl propionate to form propanoic acid |
|
The Acid Catalyzed Hydrolysis of Ethyl Esters of Aliphatic Acids
ism with esterification and saponification in that the rate constants for butyric and higher acids or esters are within experimental error |
|
Ester hydrolysis: Conditions for acid autocatalysis and a kinetic switch
May 10 2017 Keywords: autocatalysis |
|
Experiment C: Hydrolysis of a Carboxylic Acid Ester:
Figure 1: Dependence of observed hydrolysis rate constants (kh) on pH for several carboxylic acid esters. At any given pH the overall rate of ester hydrolysis |
|
Kinetics of alkaline hydrolysis of the monomethyl ester of
Experimental. The monomethyl ester of terephthalic acid was prepared from its dimethyl ester by partial hydrolysis according to the procedure described in |
|
The Hydrolysis of Esters of Some Substituted Benzoic Acids in
0.4 X 10-6 for 9.3 M sulfuric acid. The accuracy of these experiments is low and theonly conclusion of significance is that the second-orderrate coefficient. |
|
Chapter 5 Carboxylic Acids and Esters
Learn the major chemical reaction of carboxylic acids and esters and learn how to predict the products of ester synthesis and hydrolysis reactions. |
|
MIGRATION DURING HYDROLYSIS OF ESTERS OF
both glycerylphosphorylcholine and phosphatidic acid are intermediary substances. EXPERIMENTAL. Hydrolysis o.f Esters of L-a-Glycerophosphoric Acid. |
|
Mechanistic Modeling of Hydrolysis and Esterification for Biofuel
Oct 12 2011 experimental measurement of pKa for fatty acids in both water and ... ester hydrolysis in HTW and fatty acid esterification in near- or. |
|
IV SEMMESTER
EXPERIMENT Page No 1 Kinetics of Acid hydrolysis of an ester 2 2 Estimation of mixture of acids conductometrically 4 3 Estimation of Copper (II) by |
|
Experiment C: Hydrolysis of a Carboxylic Acid Ester:
Hydrolysis of a Carboxylic Acid Ester: Neutral and Base Enhanced Reaction of p -Nitrophenyl Acetate Background: The investigation of anthropogenic organic |
|
Page 1 of 12 CHEM 100L Lab 7: Ester Hydrolysis Purpose: In the
The addition of an acid catalyst can increase the rate of the reaction In this virtual experiment, you will be hydrolyzing methyl propionate by refluxing the ester in aqueous solution, using sulfuric acid as the catalyst A general mechanism for an acidic ester hydrolysis is shown below in figure 7 2 |
|
Hydrolysis of ester lab report - Squarespace
Experiment 6: Chemical equilibrium — Hydrolysis of ethyl acetate targets: ü We will study catalyzed acid (HCl) ester hydrolysis (ethyl acetate, etAc), form |
|
Experiment 5
To determine the rate constant for the acid-catalyzed hydrolysis of methyl acetate The progress of the reaction (hydrolysis of ester) is followed by removing a |
|
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
In this hydrolysis of ester (ethyl acetate) with an alkali (sodium hydroxide), HCl was used as catalyst to the presence of a mineral acid gives acetic acid and ethyl alcohol final titre value at the end of the experiment and Vt = titre value at |
|
Acid hydrolysis
products of ester hydrolysis will be alkanols plus either free alkanoic acids (at low procedure is most commonly applied to total lipid extracts, but can also be |
|
Student 3: results of 22-9-06 experiment (velocity constant):
To Determine the Velocity Constant for the Hydrolysis of Methylacetate Using an Theory: Methylacetate hydrolyses in presence of an acid (acts as catalyst) and produces Let, a = initial concentration of ester (at t = 0) of the above reaction |