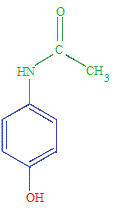
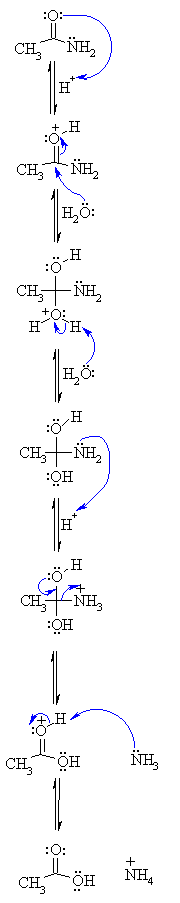
amine that results from the base hydrolysis of acetaminophen
How do you rehydrate acetaminophen?
Record the weight. Recrystallize all but 100 mg of your crude acetaminophen from water by first dissolving the solid in the minimum amount of hot (boiling) water. Do this carefully adding small amounts of hot water. You do not want to have excess water. Work on a steam bath to keep the solution hot. Add another 2 mL of hot water.
Is acetaminophen a weak acid?
Paracetamol (acetaminophen) is a weak acid. The equilibrium position lies very far to the left. The vast majority of paracetamol molecules in an aqueous solution will be found as the undissociated paracetamol molecules.
Which reaction produces an amine and a carboxylic acid?
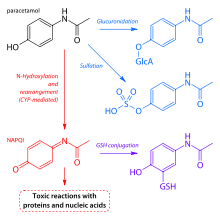
Hydrolysis (reaction with water) of amides in acidic solution produces an amine and a carboxylic acid. Hydrolysis of paracetamol (acetaminophen) in acidic solution produces an amine (4-aminophenol) and a carboxylic acid (acetic acid)
What acetaminophen is soluble in water?
The Merck Index, which is an encyclopedia of chemicals, drugs, and biologicals, lists the following information under acetaminophen: large monoclinic prisms from water, mp 169-170.5, very slightly sol in cold water, considerably more soluble in hot water. Sol in methanol, ethanol, dimethylformamide, acetone, ethyl acetate.

Primary Amine Formation/Hydrolysis reaction

Amine Synthesis Reactions

19.5 Imine and Enamine Formation Addition of Amines Organic Chemistry
|
Effect of Solution pH on the Adsorption of Paracetamol on
22 juin 2017 Abstract: Paracetamol adsorption in acidic neutral and basic ... Finally |
| Interactions and incompatibilities of pharmaceutical excipients with |
|
Studies of the Thermal Degradation of Acetaminophen Using a
undergoes either acid or base catalyzed hydrolysis in aqueous forms of acetaminophen were determined from the resulting. |
|
Rafidain Journal of Science Estimation of p-Aminophenol via
pure or the result from acidic or basic hydrolysis of paracetamol. that can interact with the two functional groups (hydroxyl and the amine) of the ... |
|
Synthesis of paracetamol by acetylation
spectrum of 4-aminophenol shows the two N-H amine bands at 3340 and 3282 cm-1 classical reactions in organic chemistry such as alkaline hydrolysis of a ... |
|
N-Dealkylation of Amines
20 mai 2022 Subsequent acid- or base-hydrolysis [22–25] or reduction [2627] of ... may undergo ring-opening resulting in the formation of a terminal ... |
|
Chapter 5 Carboxylic Acids and Esters
predict the products of ester synthesis and hydrolysis reactions. Carboxylic acids react with strong bases such as ... the trade names Tylenol. |
|
Basic analytical toxicology
General laboratory findings occur in severe poisoning with iron ethanol |
|
GENERAL TESTS PROCESSES AND APPARATUS
alkaline with sodium hydroxide TS and if the preparations 15 to 30 minutes |
| A SN1 Reaction: Synthesis of tert-Butyl Chloride |
|
Studies of the Thermal Degradation of Acetaminophen Using a
undergoes either acid or base catalyzed hydrolysis in aqueous solutions, the forms of acetaminophen were determined from the resulting mass spectra and decarboxylation and formation of the corresponding amine and is consistent with |
|
Synthesis of paracetamol by acetylation - The Royal Society of
spectrum of 4-aminophenol shows the two N-H amine bands at 3340 and 3282 cm-1, Both a qualitative and quantitative analysis of the TLC results in this classical reactions in organic chemistry such as alkaline hydrolysis of a γ-keto ester |
|
Paracetamol - CPY Document
traces of para-aminophenol, and humid conditions that cause hydrolysis genicity studies of phenacetin result in exposure of animaIs to paracetamol For the results of N-nitrosobutyl-N-(4-hydroxybutyl)amine at 0 or 0 05 (v/v) in the drinking-water assayed by alkaline elution, but the toxic metabolite of paracetamol, |
|
Opinion of the SCCP on para-Aminophenol, A16 - European
Fouramine P; Fourrine 84; Fourrine P Base; Furro P Base; Imexine OB and other aromatic amines in humans The results indicated that the epidermis transforms PAP into paracetamol standards before and after enzyme hydrolysis |
|
Paracetamol - School of Chemistry University of Leeds
Paracetamol is made by reacting 4-aminophenol with ethanoic anhydride (more commonly called acetic ethanoic acid The lone pair of electrons on the amine of 4-aminophenol attacks the C=O bond of acetic The result is an amide bond formation and a carboxylic acid by-product top of the base of the clamp stand |
|
PARACETAMOL: MECHANISM OF ACTION, APPLICATIONS AND
Abstract: Paracetamol / acetaminophen is one of the most popular and most commonly used analgesic and basis of the obtained results, a faulty conclusion was amine (4) That discovery revolutionized the phar- maceutical market of analgesic drugs and since then underwent hydrolysis to paracetamol and diethyl- |