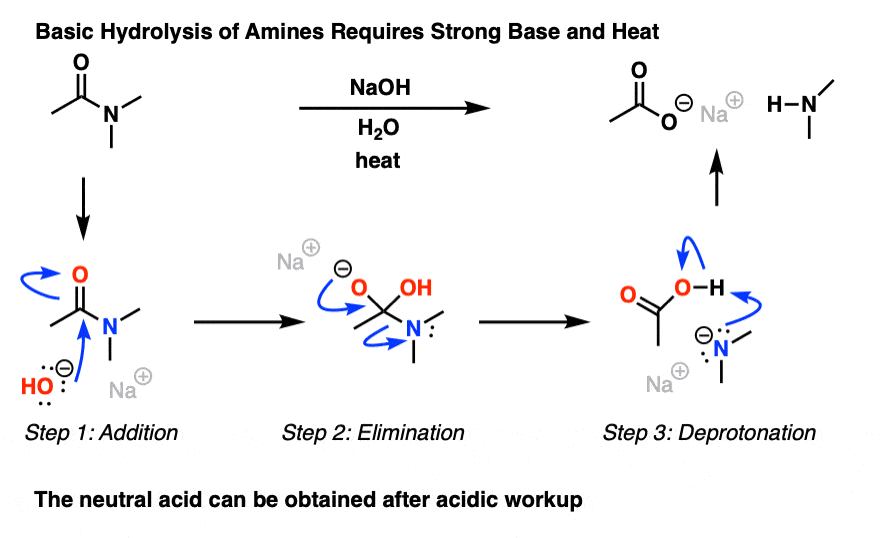
base hydrolysis of ester mechanism
How is a hydrolysis reaction reversible?
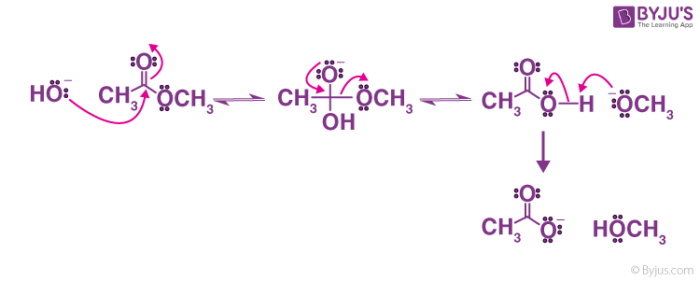
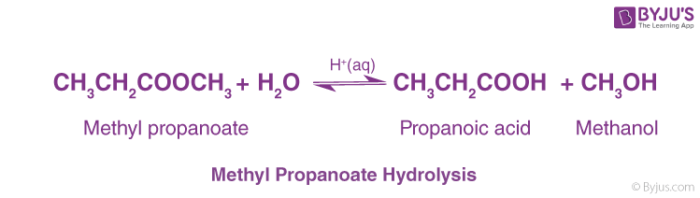
The reaction with pure water is so slow that it is never used. The reaction is catalyzed by dilute acid, and so the ester is heated under reflux with a dilute acid like dilute hydrochloric acid or dilute sulfuric acid. Here are two simple examples of hydrolysis using an acid catalyst. Notice that the reactions are reversible.
How do you hydrolyze a dilute ester?
The water comes from the dilute acid, and so you would mix the ester with an excess of dilute acid. This is the usual way of hydrolyzing esters. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution. There are two advantages of doing this rather than using a dilute acid.
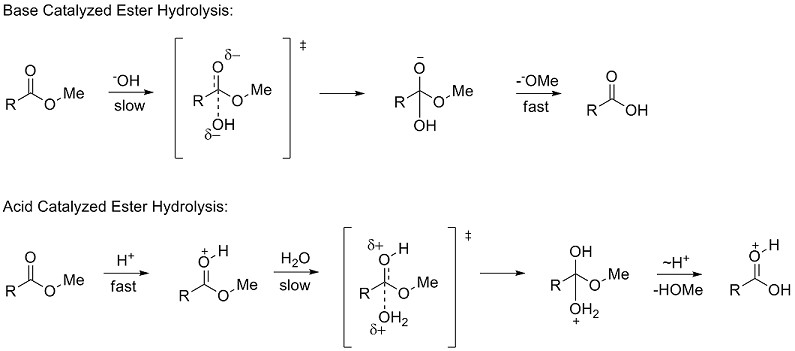
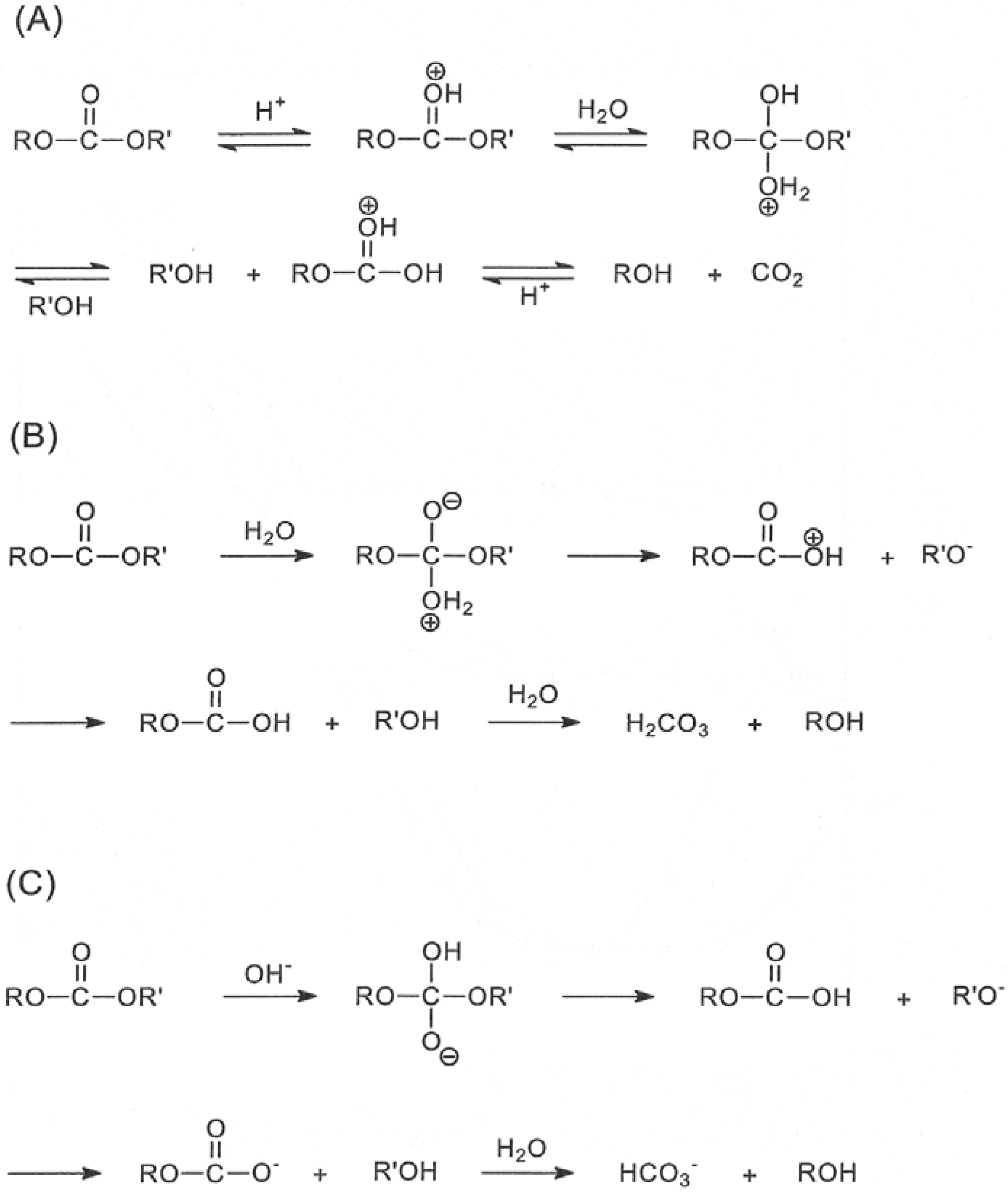
What is the mechanism of base-induced ester hydrolysis (saponification)?
Mechanism of base-induced ester hydrolysis (saponification). The mechanism shown in Figure 21.8 is supported by isotope-labeling studies. When ethyl propanoate labeled with 18 O in the ether-like oxygen is hydrolyzed in aqueous NaOH, the 18 O label shows up exclusively in the ethanol product.

Ester Hydrolysis Reaction Mechanism

Saponification

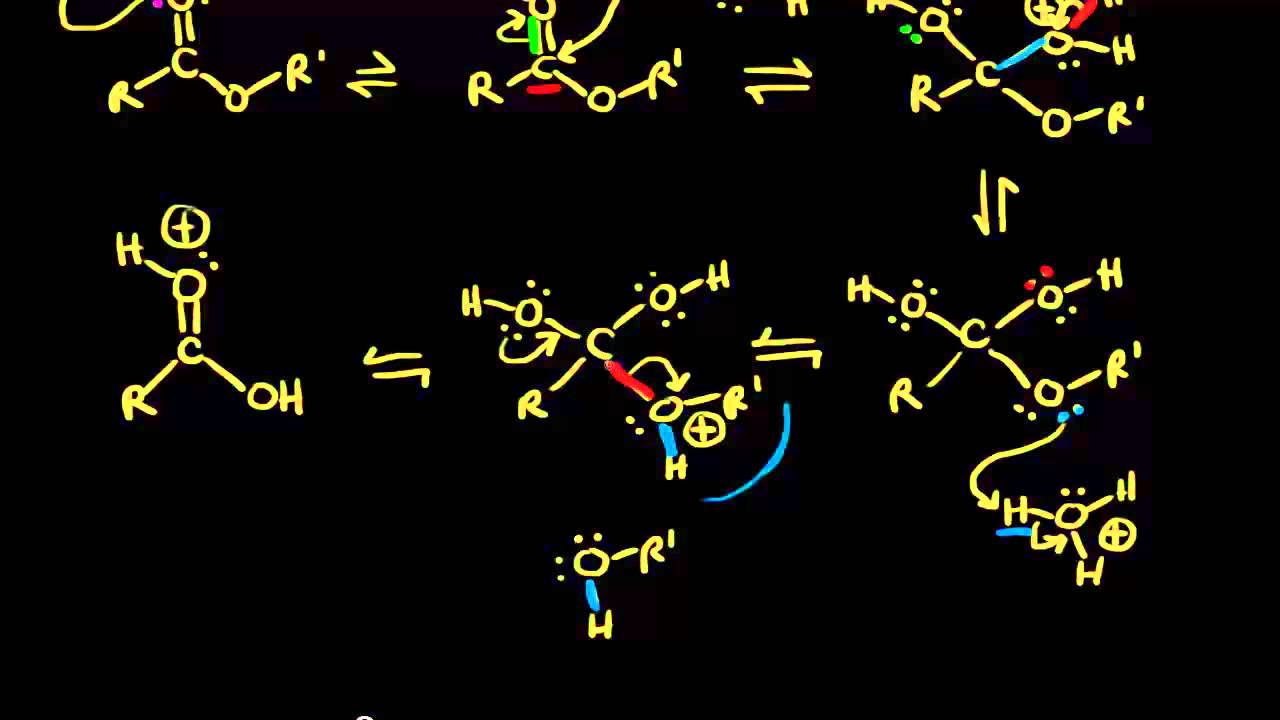
Acid-catalyzed ester hydrolysis Organic chemistry Khan Academy
|
General Basic Catalysis of Ester Hydrolysis and Its Relationship to
The action of imidazole as a general basic catalyst offers only a partial ex- planation of the mechanism of enzymatic hydrolysis. Introduction. The hydrogen and |
|
HYDROLYSIS 2016.pdf
carbon centre such as with carboxylic acid derivatives including esters |
|
General base catalysis of ester hydrolysis
Both nucleophilic and general base mechanisms of catalysis by acetate anions are observed for the hydrolysis of substituted phenyl formates with leaving |
|
A Facile Base-catalyzed Ester Hydrolysis Involving Alkyl-Oxygen
(4) base catalysis. Reaction 1 is of course the ordinary mechanism by which most esters undergo base-catalyzed hydrolysis.4 Anchimeric catalysis (2)has been. |
|
The mechanism of hydrolysis of a cobalt(III)-bound phosphate ester
The intermediate hydroxo(phosphoramido)tetraammine complex is hydrolyzed slowly by base to liberate phosphoramidate anion by the same mechanism. The ester |
|
Studies on the BAL2 mechanism for ester hydrolysis
JUDY E. DOUGLAS GRANT CAMPBELL |
|
Efficiency of lithium cations in hydrolysis reactions of esters in
31 mars 2021 6-13) These reports noted that LiOH was an effective base for the hydrolysis of esters but did not explain the mechanism. In addition many of ... |
|
The Kinetics of the Base-catalyzed Methanolysis of Ortho Meta and
Although the basic hydrolysis of esters has been the subject of numerous kinetic studies4 only one previous study of the kinetics and temperature. |
|
Cleavage of the Alkyl-Oxygen Bond in the Hydrolysis of Esters. t
mechanism11 and we should expect the /-butyl ester to show such hindrance to a high degree. To this may be ascribed the resistance to the base- catalyzed ester |
|
Carbonion (ElcB)mechanism of ester hydrolysis. I. Hydrolysis of
modes of HO~ attack on malonate esters are conceivable a priori (1 and 2a and 2b). In eq 1 the mechanism is that for normal alkaline hydrolysis of. |
|
HYDROLYSIS
mechanisms account for neutral, acid and base hydrolysis consideration of the dominant substitution mechanism corresponding di-ester derivatives |
|
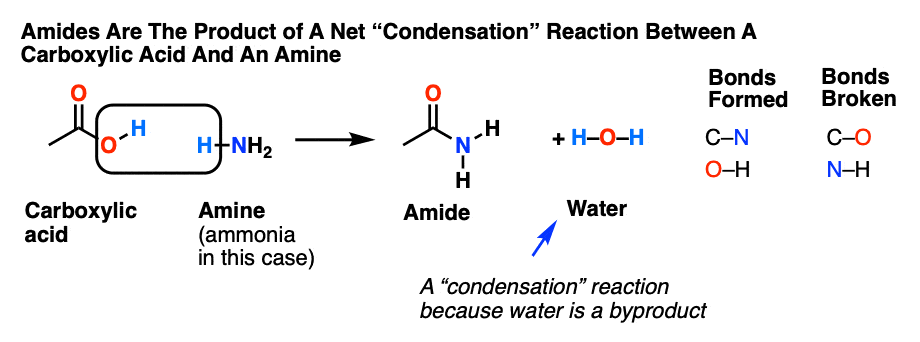
Lecture 6: Hydrolysis Reactions of Esters and Amides
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions form new esters by base- or acid-catalysed transesterification mechanisms; |
|
GENERAL BASE AND NUCLEOPHILIC CATALYSIS OF ESTER
A Mechanism Change as a Function of the Nucleophile and Leaving Group 271 anhydride In addition to general base-catalyzed hydrolysis reactions, |
|
Page 1 of 12 CHEM 100L Lab 7: Ester Hydrolysis Purpose: In the
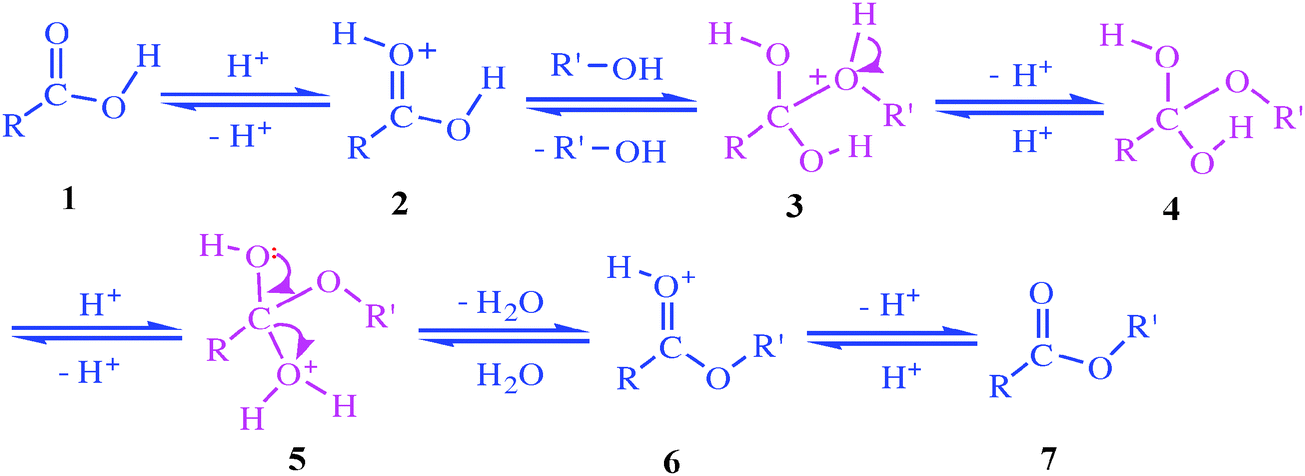
Figure 7 2 Mechanism for acid-catalyzed ester hydrolysis (From Organic Chemistry by Bruice, 8th Ed ) Once the reaction is complete, you will collect the mass |






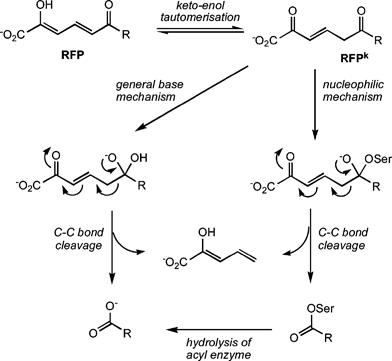
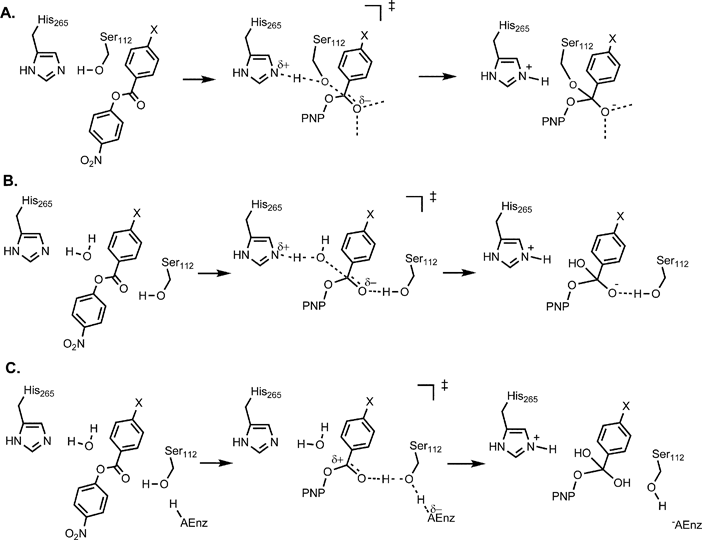









![Studies on the B AL 2 mechanism for ester hydrolysis - [PDF Document] Studies on the B AL 2 mechanism for ester hydrolysis - [PDF Document]](https://image.slidesharecdn.com/esterhydrolysis-130213014134-phpapp02/95/acid-base-catalysed-ester-hydrolysis-13-638.jpg?cb\u003d1360719744)