Lucentis, INN-ranibizumab - Europa EU
|
Lucentis INN-ranibizumab
Lucentis is indicated in adults for: • The treatment of neovascular (wet) age-related macular degeneration (AMD) • The treatment of visual impairment due |
|
Lucentis INN-ranibizumab
Lucentis est indiqué chez les adultes dans: • Le traitement de la forme néovasculaire (humide) de la dégénérescence maculaire liée à l'âge (DMLA) |
|
LUCENTIS (ranibizumab)
21 nov 2012 · LUCENTIS est un traitement de première intention dans le traitement de la baisse d'acuité visuelle due à la DMLA exsudative rétrofovéolaire La |
|
Lucentis INN-ranibizumab
Lucentis está indicado en adultos para: • El tratamiento de la degeneración macular asociada a la edad (DMAE) neovascular (exudativa) |
|
Lucentis INN
Lucentis is a medicine used to treat adults with certain conditions that impair their sight by damaging the retina (the light-sensing layer at the back of |
|
(LUCENTIS Avis1 CT4033 modifié le 30 avril 2007[1])
30 avr 2007 · Un plan de gestion des risques accompagnant la mise sur le marché du ranibizumab en Europe prévoit la surveillance particulière des effets |
|
Notice : information du patient
LUCENTIS® appartient à un groupe de médicaments appelé médicaments contre la néovascularisation Il contient un principe actif appelé |
Pourquoi LUCENTIS retiré du marché ?
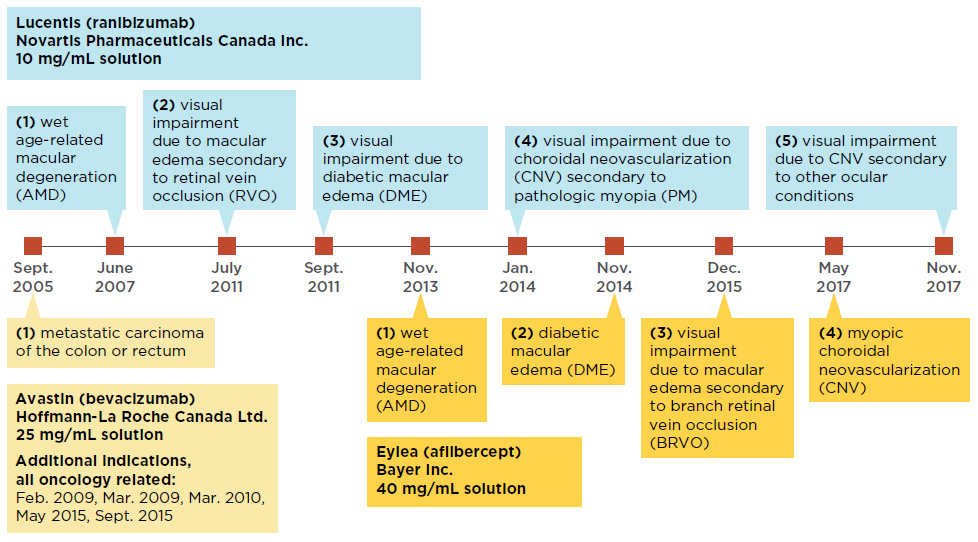
La présente affaire a pour origine la condamnation par l'Autorité de la concurrence des laboratoires Roche, Novartis et Genentech pour avoir mis en œuvre des pratiques abusives visant à préserver les ventes du médicament Lucentis pour le traitement de la DMLA (dégénérescence maculaire liée à l'âge) au détriment d'
Quel est le prix de LUCENTIS ?
Présentations du médicament LUCENTIS
Sur ordonnance (Liste I), médicament à prescription restreinte, médicament d'exception - Remboursable à 100 % - Prix : 433,55 €.
Les prix mentionnés ne tiennent pas compte des « honoraires de dispensation » du pharmacien.Quelle est la différence entre Eylea et LUCENTIS ?
EYLEA diffère de LUCENTIS par un schéma d'administration fixe la première année (trois injections mensuelles puis une tous les 2 mois) et par un suivi tous les 2 mois.
Les données d'efficacité et de tolérance n'ont pas mis en évidence d'avantage clinique par rapport à LUCENTIS.LUCENTIS® est administré par votre ophtalmologiste, sous forme d'une injection unique, dans l'œil, sous anesthésie locale.
Le traitement sera initié avec une injection par mois.
|
Lucentis INN-ranibizumab
Lucentis est indiqué chez les adultes dans: •. Le traitement de la forme néovasculaire (humide) de la dégénérescence maculaire liée à l'âge. (DMLA). |
|
Lucentis INN-ranibizumab
European Medicines Agency 2018. Reproduction is authorised provided the source is acknowledged. EMA/554038/2018. EMEA/H/C/000715. Lucentis (ranibizumab). |
|
Lucentis INN-ranibizumab
Detailed information on this medicinal product is available on the website of the European Medicines. Agency http://www.ema.europa.eu. Page 28. 28. 1. NAME OF |
|
Ranivisio INN-ranibizumab
Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 études sur l'efficacité et la sécurité du ranibizumab menées sur Lucentis. |
|
Byooviz INN-Ranibizumab
Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 études sur l'efficacité et la sécurité du ranibizumab menées sur Lucentis. |
|
Luncentis INN-Ranibizumab
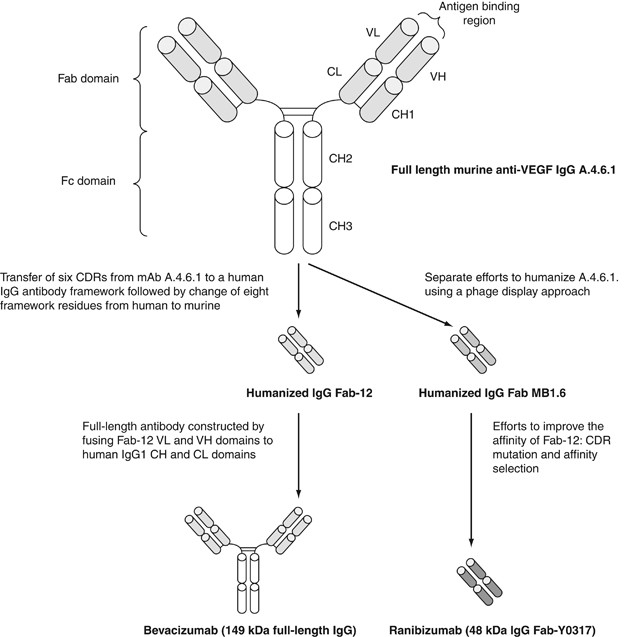
Lucentis is a new medicinal product containing a biotechnology-derived protein (ranibizumab) as drug substance. Ranibizumab is a humanised monoclonal antibody |
|
Lucentis INN-ranibizumab
European Medicines Agency 2018. Reproduction is authorised provided the source is acknowledged. EMA/554038/2018. EMEA/H/C/000715. Lucentis (ranibizumab). |
|
Lucentis INN-ranibizumab
20 août 2010 Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000. Lucentis. Procedural steps taken and scientific information ... |
|
Lucentis INN-ranibizumab
19 sept. 2019 Send us a question Go to www.ema.europa.eu/contact Telephone +31 ... For information the full indication for Lucentis will be as follows:2. |
|
Lucentis INN-ranibizumab
30 mai 2013 EMA/CHMP/320328/2013. Committee for Medicinal Products for Human Use (CHMP). Summary of opinion1 (post authorisation). Lucentis ranibizumab. |
|
Lucentis, INN-ranibizumab - europaeu
Lucentis 10 mg/ml solution injectable 2 COMPOSITION QUALITATIVE ET QUANTITATIVE Un ml contient 10 mg de ranibizumab* Chaque flacon contient 2,3 |
|
Lucentis, INN-ranibizumab
Lucentis is used in adults to treat several eye diseases causing vision Detailed information on this medicine is available on the European Medicines Agency |
|
Lucentis - WHO World Health Organization
28 nov 2014 · 4 International Nonproprietary Name (INN, generic name) of the Information as provided in the approved ranibizumab (Lucentis®) EU SmPC, |
|
Lucentis, INN - ranibizumab
Send a question via our website www ema europa eu/contact Lucentis ranibizumab This is a summary of the European public assessment report ( EPAR) for |
|
Efficacité du traitement par injections intravitréennes de - CORE
22 avr 2016 · entific research documents, whether they are pub- lished or not Ranibizumab dans l'œdème maculaire diabétique Présentée et soutenue publiquement chirurgical que j'ai eu le plaisir de découvrir dans votre service (Lucentis ®, Novartis, Bâle, Suisse) a été le premier anti-VEGF à obtenir l'AMM en |
|
Lucentis, INN - ranibizumab - Medicina e Società
ranibizumab Questo è il riassunto della relazione pubblica europea di valutazione (EPAR) per Lucentis Illustra il modo in cui l'Agenzia ha valutato il medicinale |
|
Lucentis, INN-ranibizumab - Novartis
Jede Durchstechflasche enthält 2,3 mg Ranibizumab in 0,23 ml Lösung worden sind Lucentis und Photodynamische Therapie mit Verteporfin bei CNV aufgrund einer PM Arzneimittel-Agentur http://www ema europa eu/ verfügbar |





![Full text] Initiation and maintenance of a Treat-and-Extend Full text] Initiation and maintenance of a Treat-and-Extend](https://els-jbs-prod-cdn.jbs.elsevierhealth.com/cms/attachment/eb4252e4-b88b-4763-96d4-21eeba05bc6c/gr1.jpg)


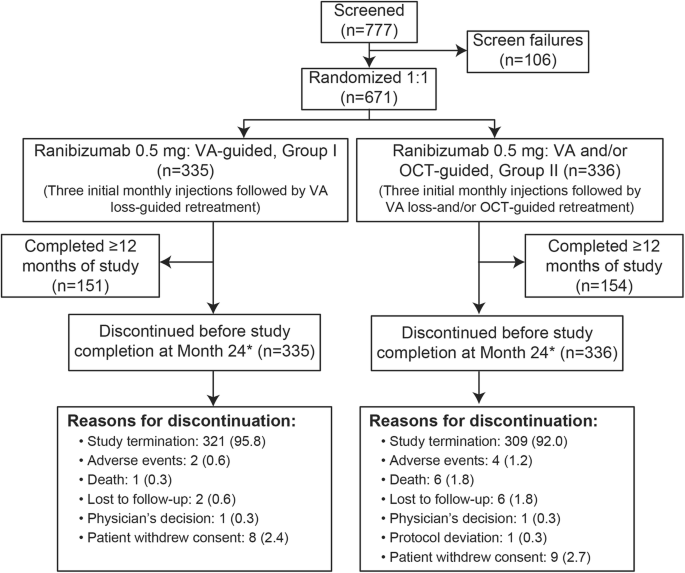






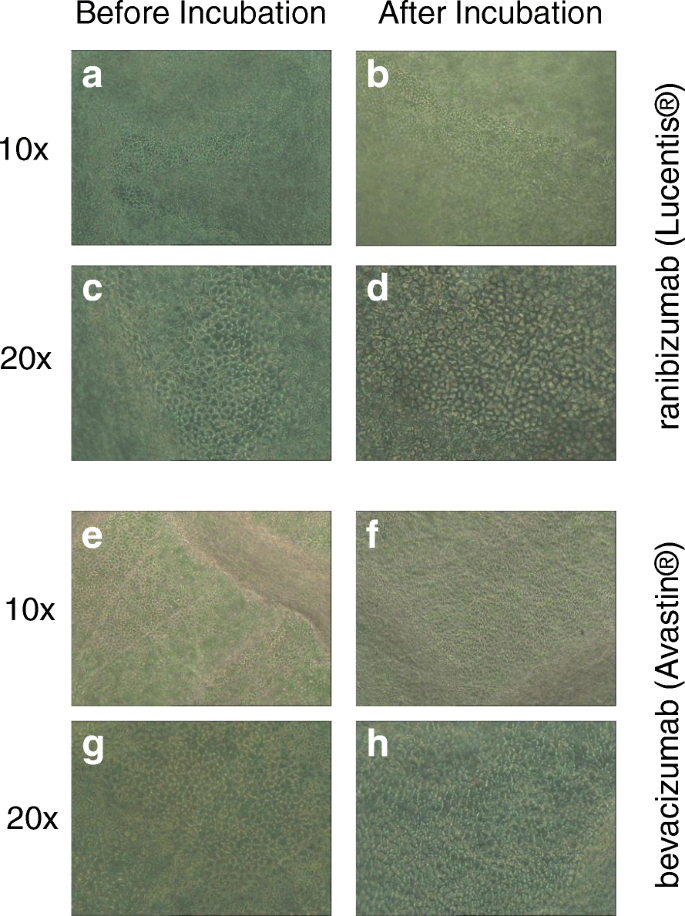




![Full text] Cost-effectiveness of ranibizumab versus aflibercept in Full text] Cost-effectiveness of ranibizumab versus aflibercept in](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Feye.2016.269/MediaObjects/41433_2017_Article_BFeye2016269_Fig1_HTML.jpg)