Lucentis, INN - ranibizumab - European Medicines Agency - Europa
|
Lucentis INN-ranibizumab
Lucentis is indicated in adults for: • The treatment of neovascular (wet) age-related macular degeneration (AMD) • The treatment of visual impairment due |
|
Lucentis (ranibizumab)
Lucentis is a medicine used to treat adults with certain sight problems caused by damage to the retina (the light-sensing layer at the back of the eye) |
|
biosimilars
Information about specific biosimilar medicines is available from a number of sources including the European Medicines Agency (EMA) and this should aid |
|
The Impact of Biosimilar Competition in Europe
The European Medicines Agency (EMA) has a central role in Eylea (aflibercept) and Lucentis (ranibizumab) are anti-VEGF agents used to treat several ocular |
|
Extensions of indication in the European Union – a regulatory
European Medicines Agency Retrieved on 11/04/2016 from http://www ema europa eu/docs/en_GB/document_library/Other/2016/03/ WC500203971 pdf (29) EMA (27 |
|
Transparency of Regulatory Data across the European Medicines
Historically sponsors and regulatory agencies have kept confidential much of the clinical data generated to support the approval and |
Is LUCENTIS FDA approved?
LUCENTIS was the first FDA-approved anti-VEGF medicine for diabetic retinopathy (DR) with or without diabetic macular edema (DME)
What is the active ingredient in ranibizumab?
Lucentis belongs to a group of medicines called antineovascularisation agents.
It contains the active substance called ranibizumab.
Lucentis is used in adults to treat several eye diseases causing vision impairment.What pharmaceutical company makes LUCENTIS?
Genentech: Lucentis® (ranibizumab injection) - Information for Patients.
Ranibizumab (Lucentis).
This drug was approved for wet AMD in 2006.
A dose costs around $1,800-$2,000 before insurance.
|
Lucentis INN-ranibizumab
Detailed information on this medicinal product is available on the website of the European Medicines. Agency http://www.ema.europa.eu. Page 28. 28. 1. NAME OF |
|
Lucentis INN-ranibizumab
Send a question via our website www.ema.europa.eu/contact The active substance in Lucentis ranibizumab |
|
Lucentis INN-ranibizumab
25 juil. 2019 Diabetic macular edema. EMA. European Medicines Agency. ETROP. Early Treatment for Retinopathy of Prematurity trial. EU. European Union. |
|
Lucentis INN - ranibizumab
13 oct. 2016 Send a question via our website www.ema.europa.eu/contact ... Lucentis (ranibizumab) has been approved in the European Union (EU)/ European ... |
|
Lucentis INN-ranibizumab
19 sept. 2019 Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 ... Lucentis® (ranibizumab) is a recombinant humanised immunoglobulin ... |
|
Luncentis INN-Ranibizumab
The last inspection of the drug substance and drug product manufacturing facilities showed compliance to EU cGMP. Page 8. 8/54. ©EMEA 2007. Except for a number |
|
Lucentis INN-ranibizumab
19 sept. 2019 Send us a question Go to www.ema.europa.eu/contact Telephone +31 ... For information the full indication for Lucentis will be as follows:2. |
|
Lucentis INN-ranibizumab
30 mai 2013 An agency of the European Union ... E-mail info@ema.europa.eu Website www.ema.europa.eu. © European Medicines Agency 2013. |
|
Lucentis INN-ranibizumab
30 mai 2013 EMA/716504/2012. Page 7/60. Lucentis (ranibizumab) was approved in the EU/EEA in 2007 via the centralised procedure for the. |
|
Lucentis INN-ranibizumab
21 oct. 2010 E-mail info@ema.europa.eu Website www.ema.europa.eu ... Ranibizumab (Lucentis) is a recombinant humanised IgG1 ? isotype monoclonal antibody ... |
|
Lucentis, INN-ranibizumab - European Medicines Agency - europa
EMEA/H/C/000715 Lucentis (ranibizumab) An overview of Lucentis and why it is authorised in the EU What is Lucentis and what is it used for? Lucentis is a |
|
Lucentis, INN-ranibizumab - European Medicines Agency - europa
Adults The recommended dose for Lucentis in adults is 0 5 mg given as a single intravitreal injection This corresponds to an injection volume of 0 05 ml The |
|
Lucentis, INN-ranibizumab
Read all of this leaflet carefully before you are given this medicine because it contains important information for you Detailed information on this medicine is available on the European Medicines Agency website: http://www ema europa eu |
|
Lucentis - WHO World Health Organization
28 nov 2014 · 4 International Nonproprietary Name (INN, generic name) of the medicine 4 injection to the WHO's list of essential eye medicines 2 Information as provided in the approved ranibizumab (Lucentis®) EU SmPC, dated Sep http://www ema europa eu/docs/en_GB/document_library/EPAR_- |
|
Biosimilar medicines - Medicines For Europe
Medicines for Europe and represents the leading companies developing Although the European Medicines Agency The INN is also approved by the EMA during Ranibizumab Lucentis® Antivascular endothelial growth factor ( VEGF) |
|
The Panel - ISPOR
medicine Product INN Number of biosimilar versions licensed in Europe European Medicines Agency, Biosimilar Medicines (ranibizumab) Lucentis |
|
Extensions of indication in the European Union – a - DGRA
EMA European Medicines Agency EPAR European public assessment report EPAR research be considered for any particular EU member state (13) Compared to the product name of the existing MA, the INN of the MP “will be the same for 27/05/2015 tolvaptan See MA date X Lucentis 22/01/2007 ranibizumab |
|
INFORMATION - Parallels Plesk Panel
Ranibizumab and stroke 14 The harmonization of regulatory requirements between Europe, Japan and the USA ment guideline is expected to be pub- ( Lucentis®) has advised healthcare registered by the National Drug Authority |






![Full text] Cost-effectiveness of ranibizumab versus aflibercept in Full text] Cost-effectiveness of ranibizumab versus aflibercept in](https://imgv2-1-f.scribdassets.com/img/document/361560226/original/b2e03edb22/1610081596?v\u003d1)



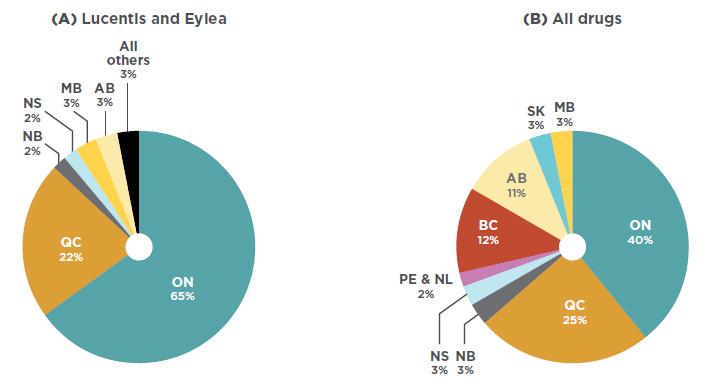





![[Full text] Cost-effectiveness of ranibizumab versus aflibercept in the [Full text] Cost-effectiveness of ranibizumab versus aflibercept in the](https://els-jbs-prod-cdn.jbs.elsevierhealth.com/cms/attachment/0a49b140-72f0-49a1-8103-f04e2f57910b/gr1.jpg)



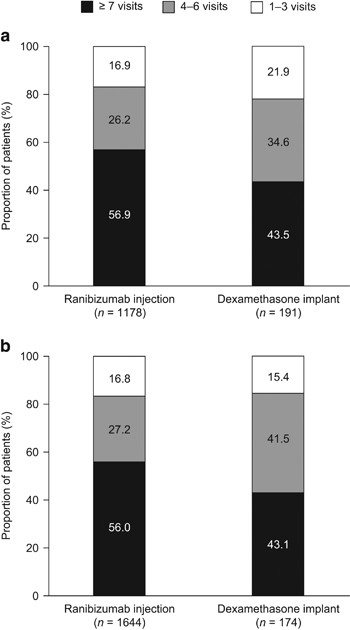

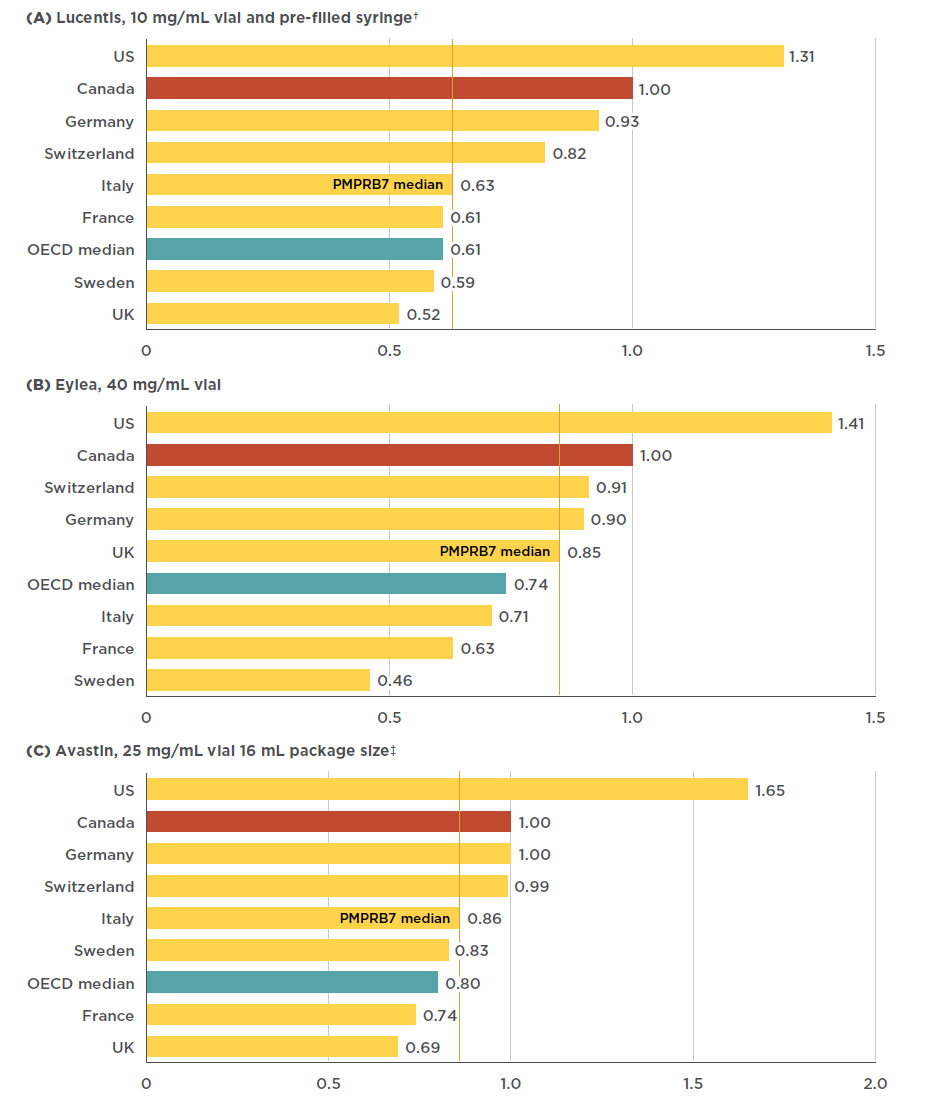


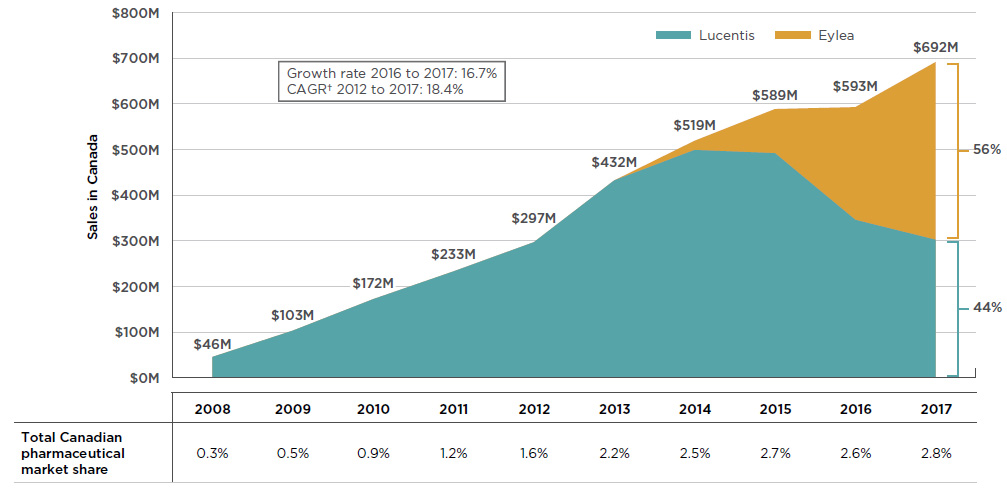
![Full text] Cost-effectiveness of ranibizumab versus aflibercept in Full text] Cost-effectiveness of ranibizumab versus aflibercept in](http://www.pmprb-cepmb.gc.ca/CMImages/npduis/MarketIntelligence_2017/fig2-3e.jpg)





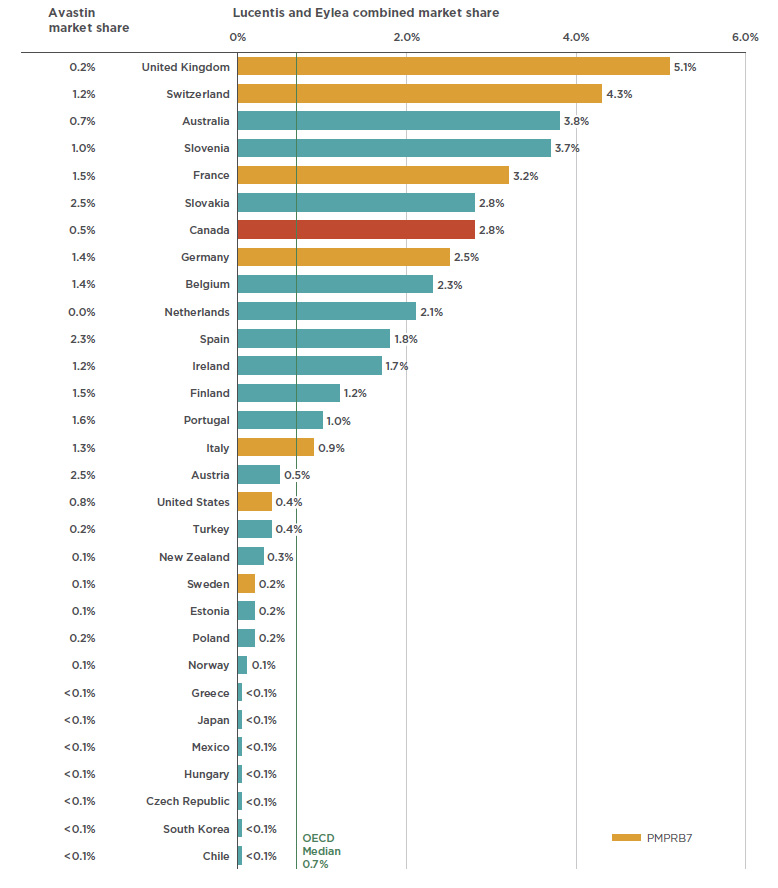
![Full text] Cost-effectiveness of ranibizumab versus aflibercept in Full text] Cost-effectiveness of ranibizumab versus aflibercept in](https://i1.rgstatic.net/publication/326589874_Neovascular_Age-Related_Macular_Degeneration_A_Visual_Acuity_Model_of_Natural_Disease_Progression_and_Ranibizumab_Treatment_Effect/links/5b58aa13a6fdccf0b2f48417/largepreview.png)






