Ofev, INN-nintedanib - European Medicines Agency - Europa EU
|
Ofev 150 mg soft capsules
The safety and efficacy of nintedanib have not been investigated in patients with hepatic impairment classified as Child Pugh B and C Treatment of patients |
|
Ofev (nintedanib)
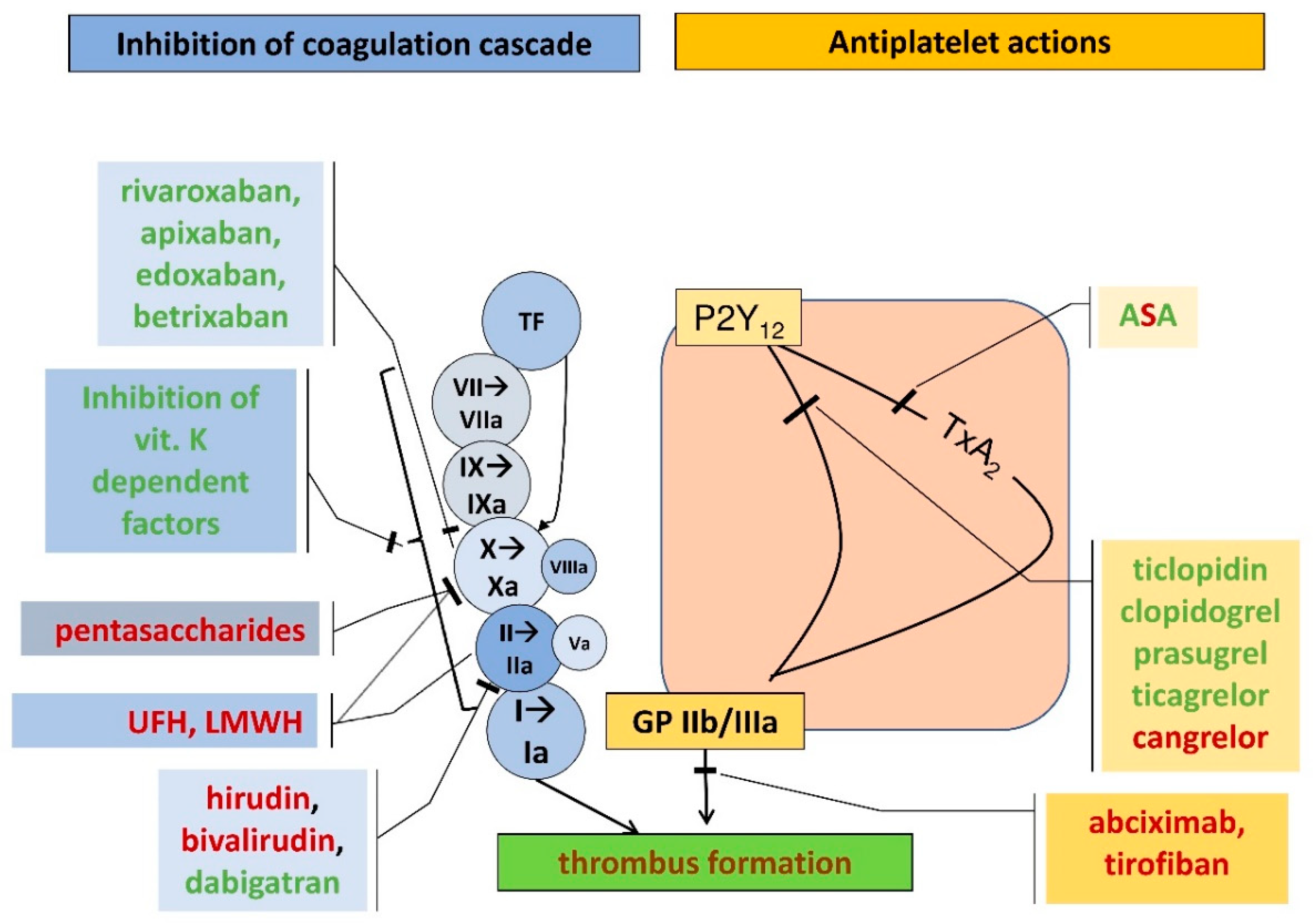
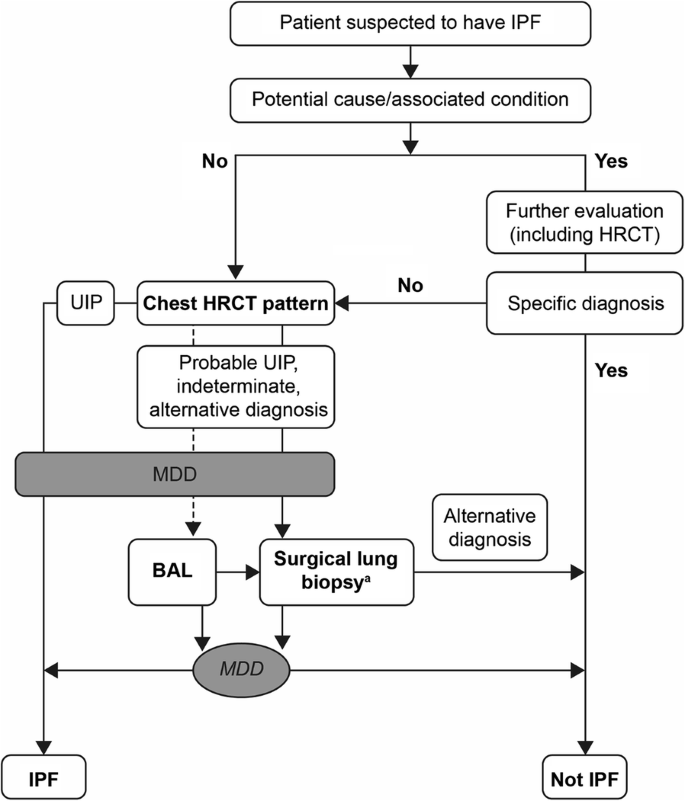
Ofev is a medicine used to treat adults with: • idiopathic pulmonary fibrosis (IPF) a disease of unknown cause in which fibrous tissue forms in the lungs; • |
|
Ofev INN-nintedanib
Detailed information on this medicinal product is available on the website of the European Medicines Agency http://www ema europa eu Page 58 29 ANNEX II A |
|
nintedanib 100mg and 150mg soft capsules (Ofev®)
7 jui 2021 · Dosing Information Nintedanib 150mg orally twice daily administered with food approximately 12 hours apart |
|
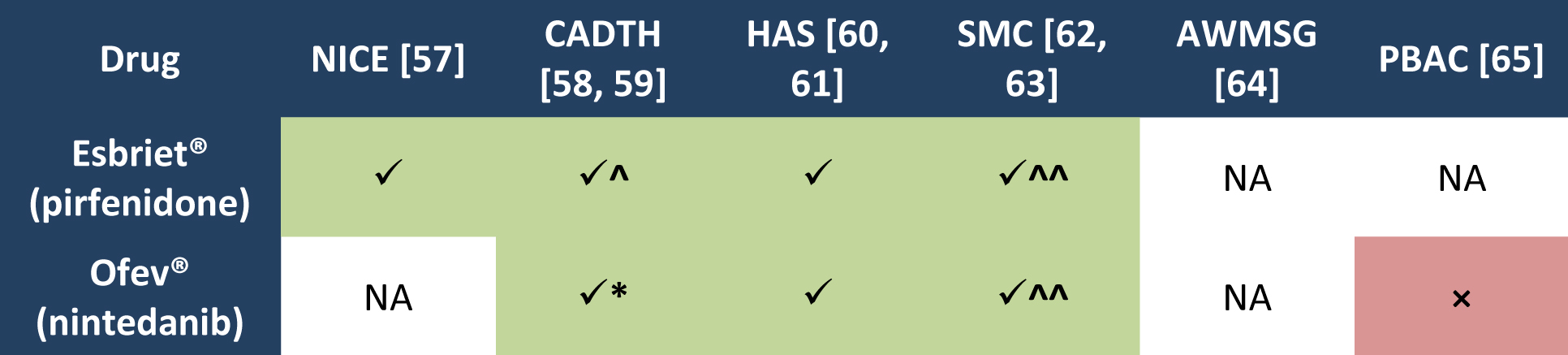
Extensions of indication in the European Union – a regulatory
European Medicines Agency Retrieved on 11/04/2016 from http://www ema europa eu/docs/en_GB/document_library/Other/2016/03/ WC500203971 pdf (29) EMA (27 |
|
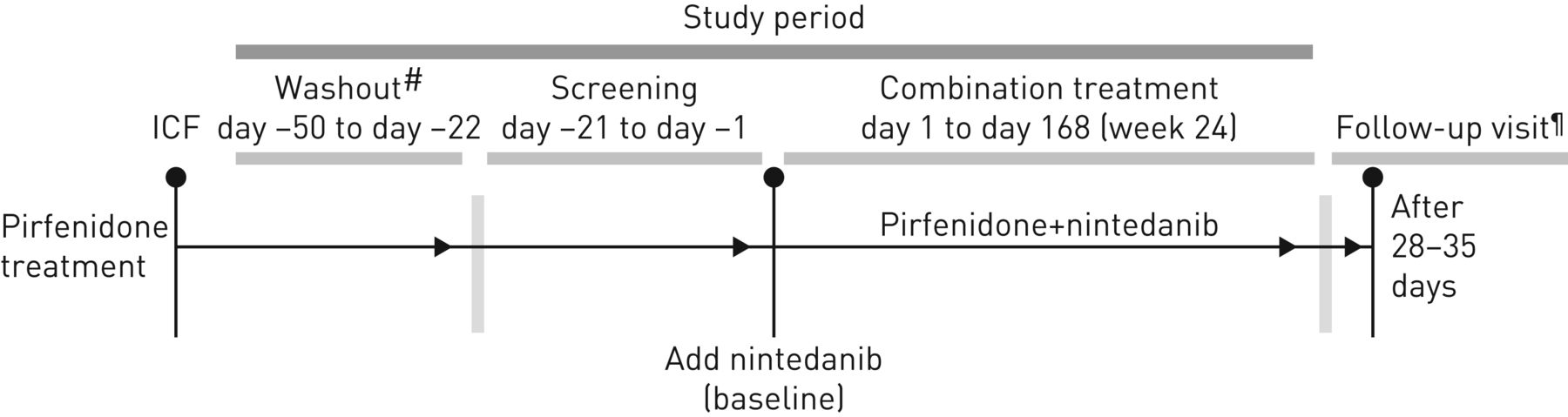
Nintedanib for Systemic Sclerosis Associated Interstitial Lung Disease
15 jan 2018 · It is given by mouth as capsules Nintedanib (as OFEV) is already available in the EU for the treatment of idiopathic pulmonary fibrosis (IPF) |
How much does nintedanib cost per month?
How to Get Started On OFEV (nintedanib) OFEV (nintedanib) is an expensive specialty medication.
A prescription may cost up to $96,000 per year or $8,000 per month.Where is nintedanib approved?
Nintedanib was approved for idiopathic pulmonary fibrosis on 15 October 2014, by the United States Food and Drug Administration (FDA), and received a positive opinion from the European Medicines Agency on 20 November 2014, being approved in the European Union in January 2015.
Why is Ofev so expensive?
Many years of testing and research must occur to make sure a brand-name drug such as Ofev is safe and effective.
This can make these drugs expensive.
There's currently no generic version available for Ofev, and there likely won't be one until 2029.Ofev® Indicated for Idiopathic Pulmonary Fibrosis (IPF) Boehringer Ingelheim.
|
Ofev INN-nintedanib
Ofev 150 mg soft capsules are brown-coloured opaque |
|
Ofev INN-nintedanib
Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 La substance active d'Ofev le nintédanib |
|
Ofev INN-nintedanib
20 nov. 2014 The outcome of the COMP review can be found on the Agency's website: ema.europa.eu/Find medicine/Rare disease designations. The legal basis for ... |
|
OFEV INN-Nintedanib
including in its plain-language summary available on the EMA website |
|
Ofev INN-nintedanib
28 mai 2020 Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 ... Ofev nintedanib. On 28 May 2020 the Committee for Medicinal ... |
|
Ofev INN-nintedanib
Further information on Ofev can be found on the Agency's website: ema.europa.eu/medicines/human/EPAR/ofev. This overview was last updated in 06-2020. |
|
Ofev INN-Nintedanib
27 fév. 2020 Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 ... Nintedanib is licenced as Ofev for the treatment of Idiopathic ... |
|
Ofev INN-nintedanib
28 mai 2020 Send us a question Go to www.ema.europa.eu/contact ... OFEV. International non-proprietary name: nintedanib. Procedure No. |
|
OFEV INN-nintedanib (respiratory indication)
Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Active substance(s): nintedanib (respiratory indication). Procedure No. |
|
Ofev INN-nintedanib
Send a question via our website www.ema.europa.eu/contact Taking into account the PRAC Assessment Report on the PSUR for nintedanib (respiratory ... |
|
Ofev, INN-nintedanib - European Medicines Agency - europaeu
EMEA/H/C/003821 Ofev (nintedanib) An overview of Ofev and why it is authorised in the EU What is Ofev and what is it used for? Ofev is a medicine used to |
|
Ofev, INN-Nintedanib - European Medicines Agency
Ofev 150 mg soft capsules are brown-coloured, opaque, oblong soft-gelatin capsules imprinted on one Cases of drug-induced liver injury have been observed with nintedanib treatment, including severe Agency http://www ema europa eu |
|
Ofev, INN-Nintedanib - Cyendiv ® (nintedanib)
Ofev is indicated in adults for the treatment of Idiopathic Pulmonary Fibrosis (IPF) 4 2 Posology and The European Medicines Agency has waived the obligation to submit the results of studies with Ofev Agency http://www ema europa eu |
|
Vargatef, INN-Nintedanib - InOncology
Recommended dose adjustments for Vargatef (nintedanib) in case of diarrhoea, The European Medicines Agency has waived the obligation to submit the results of studies with Agency http://www ema europa eu As part of Ofev PSUSA/00010319/201510, pancreatitis was added as a new adverse drug reaction |
|
Extensions of indication in the European Union – a - DGRA
Example 5 API: nintedanib EMA European Medicines Agency EPAR European public assessment be considered for any particular EU member state (13) Compared to the product name of the existing MA, the INN of the MP “will be the 15/01/2015, the MAH was granted the MA for the OMP Ofev 100 mg soft |
|
WHO Drug Information - WHO World Health Organization
9 mar 2018 · 89 Recommended INN: List 79 EMA European Medicines Agency (www ema europa eu) EU nintedanib (Ofev®) in the post-marketing |
|
Orphan Drugs versus Ultra-Orphan Drugs - ISPOR
1 European Medicines Agency (EMA) Orphan drugs and rare diseases at a glance 2016 Jul 13; Available at http://www ema europa eu/docs/en_GB/ |
|
Ofev, INN-nintedanib - Medicina e Società
Send a question via our website www ema europa eu/contact Ofev nintedanib Ofev è un medicinale impiegato negli adulti per il trattamento della fibrosi |