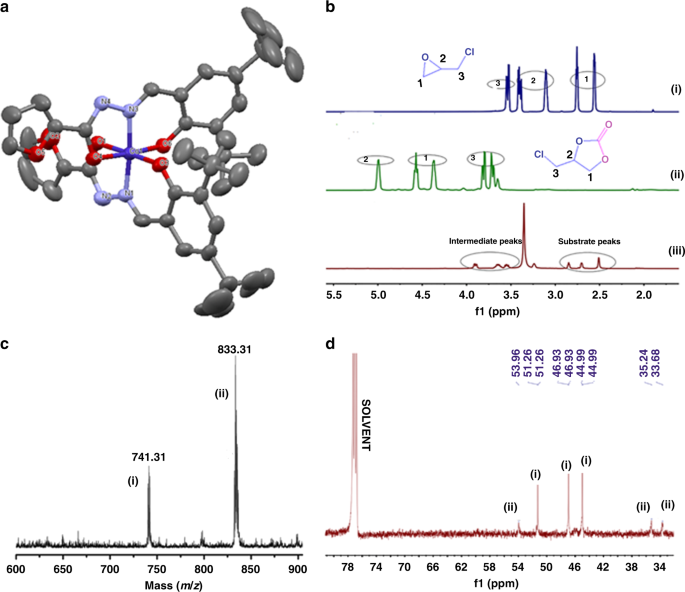
acid hydrolysis in octahedral complexes
|
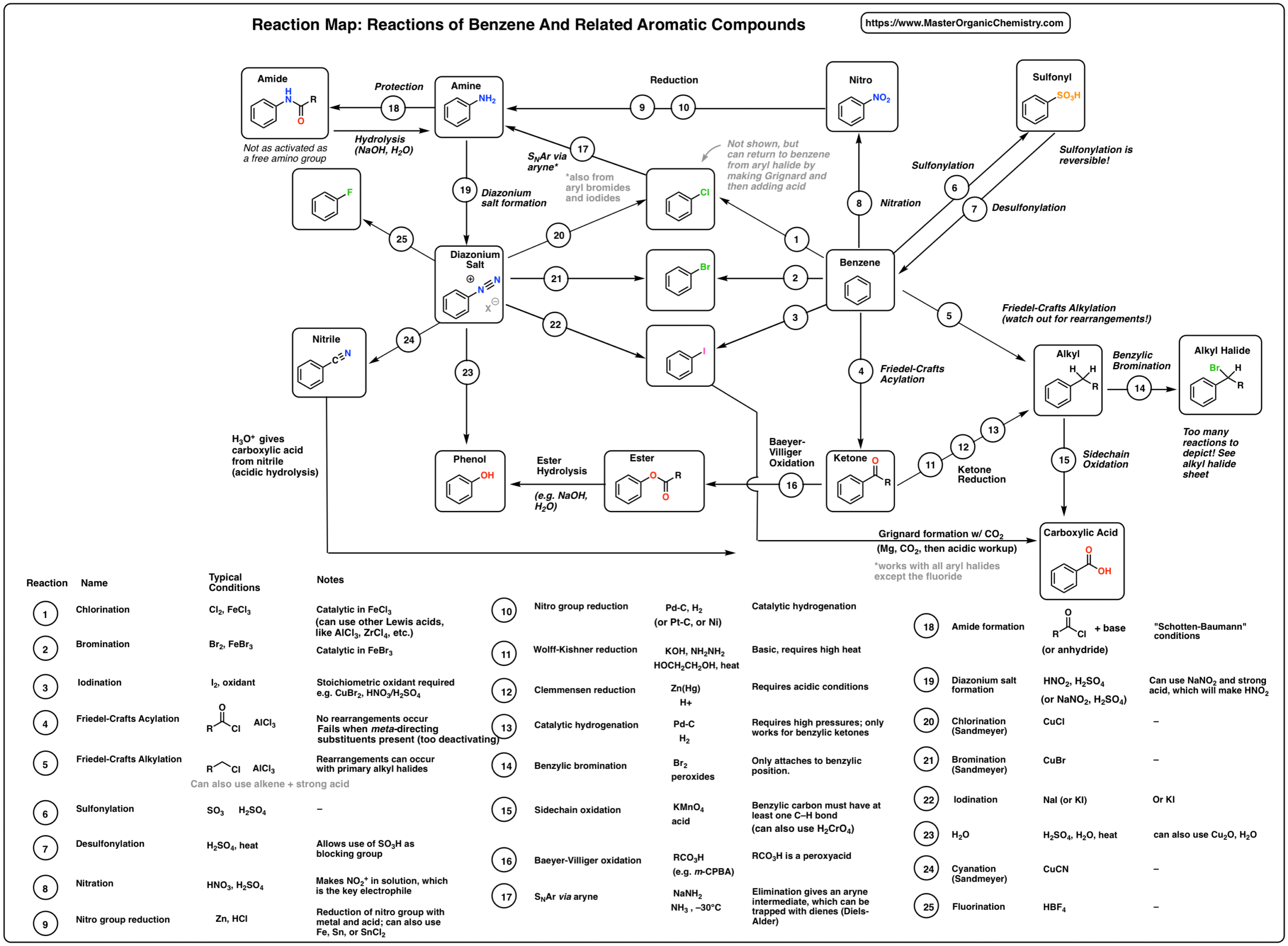
Acid Hydrolysis of Co(III) amine complexes
This experimental data supports SNICB mechanism Substitution Reactions in Square Planer complexes The majority of square planar complexes contain d8 metal |
|
Mechanism of Hydrolysis reaction
The value of rates of acid hydrolysis of some Co(III) complexes at pH=1 shows that the divalent monochloro complexes react about 100 times slower than the |
|
Substitution reactions in octahedral complexes
Acid hydrolysis: The most common reaction of a metal complex is the reaction between the complex and the solvent water The reactions of these complexes with |
|
Substitution reactions in octahedral complexes
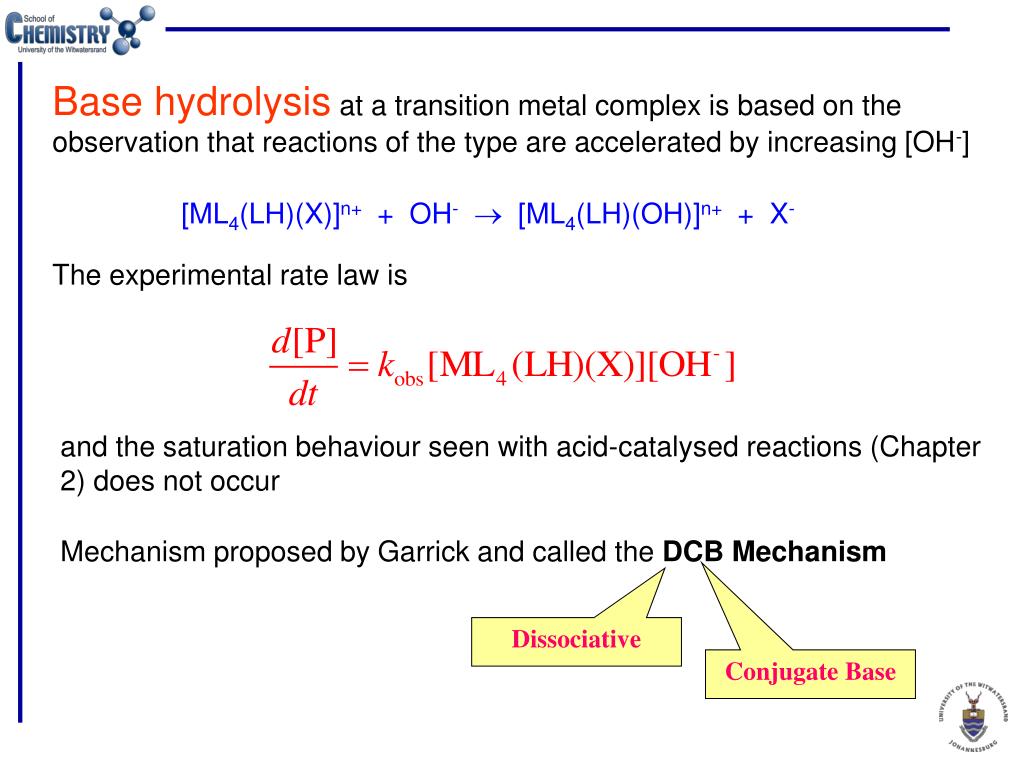
Whereas the acid hydrolysis is occurred for reactions of metal complexes with water at pH < 3 base hydrolysis occurs in basic solutions (at pH > 10) |
What are the factors affecting acid hydrolysis?
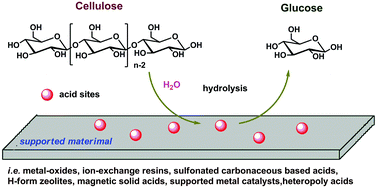
Factors influencing dilute acid hydrolysis include the degree of grinding of the cellulosic feedstock, liquid-to-solid ratio, reaction temperature, reaction time, acid concentrations, and co-catalyst type and concentration.
In the dissociative mechanism, a ligand is lost from the complex to give an intermediate compound of lower coordination number.
This type of reaction path is typical of octahedral complexes, many aqua complexes, and metal carbonyls such as tetracarbonylnickel.
What is the acid hydrolysis reaction?
Acid hydrolysis is a hydrolysis mechanism in organic chemistry in which protic acid is used to catalyse the cleavage of a chemical bond by means of a nucleophilic replacement reaction with the addition of water elements (H2O).
What is the reaction mechanism of octahedral complexes?
Two types of mechanisms are followed for base hydrolysis in octahedral complexes. (i) SN2 or Associative mechanism. (ii) SN1CB or Dissociation mechanism. sulphoxide, can be explained by SN1CB mehanism but not by SN2.
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is formed due to the replacement of a ligand by water molecule. |
|
BSc Chemistry
Acid hydrolysis of Octahedral Complex hydrolysis reactions; the water containing complex is obtained in acidic solution while the hydroxo (HO-) complex ... |
|
Substitution reactions in octahedral complexes
Whereas the acid hydrolysis is occurred for reactions of metal complexes with water at pH < 3 base hydrolysis occurs in basic solutions (at pH > 10). |
|
M.Sc Semister I Paper I Inorganic Chemistry Department of
two types (a) when an aqua complex is formed by the replacement of a ligand by H2O molecules are called acid hydrolysis or equation reactions. |
|
Reaction Mechanism of Transition Metal Complexes – I
Octahedral complexes react either by SN1 or SN2 mechanism in which the Ligand Displacement Reactions in Octahedral Complexes- Acid Hydrolysis. |
|
Acid Hydrolysis of Co(III) amine complexes
The values of rates of acid hydrolysis of some Co(III) complexes at pH= 8.5.3 Examples of Substitution Reactions in octahedral complexes. Hydrolysis ... |
|
Kinetics and mechanism of the base hydrolysis of some trans
In general these octahedral complexes undergo acid hydrolysis and substitution reactions with other ligands at rates which are of the same magnitude and which |
|
The Hydrolysis of Octahedral Complexes of Arsenic(V): The Kinetics
Hydrolysis of Octahedral Complexes of Arsenic(V). 2841 rr. Fig. 2.—The equivalent conductances of HN03 the arsenic(V)-catechol complex |
|
Mechanism of Substitution Reactions of Complex Ions. X. ?-Bonding
-BoNDiNG in Dissociation Reactions of Octahedral Complexes. 4879 an acid of Coen3+3 cannot be the rate of acid hydrolysis of cobalt(III) com- plexes.1. |
|
M.Sc. Chemistry Sem 1 Paper -102(Inorganic) Model Question 1 I
Stable complex is formed by metals which have: (a) Small size (b) High charge (c) High Acid hydrolysis of octahedral complexes proceeds by:. |
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is formed due to the replacement of a ligand by water molecule Now, it has also been found experimentally that divalent monochloro complexes of Co(III) react at much slower than monovalent dichloro complexes |
|
Kinetics of the acid hydrolysis (aquation) - CORE
It was hoped that this investigation would clarify the mechanism of substitution for reactions involving square planar platinum(II) complexes For this study, |
|
B Base Hydrolysis 25 - ScienceDirectcom
Octahedral Complexes A ACID HYDROLYSIS The most common reaction of a metal complex, and the one studied to the greatest extent, is the reaction |
|
BASE HYDROLYSIS OF COBALT(III) - ACS HIST
for the base hydrolysis of cobalt(III) ammines It is pri- stitution of octahedral metal complexes, but perhaps it of chlorosuccinic acid to form malic acid (Eq 1 ) |
|
MECHANISMS OF AQUATION AND BASE HYDROLYSIS IN
shown that when base hydrolysis of complexes of the types one or more substituents by water, the acid hydrolysis th metal ions such as TlJ+ and Ag+ |