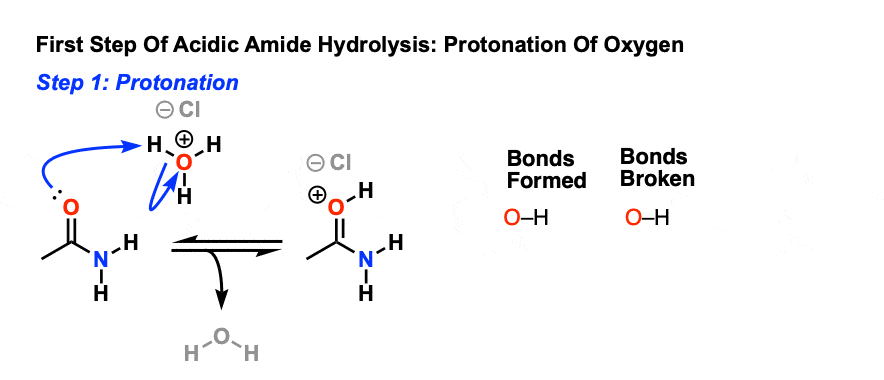
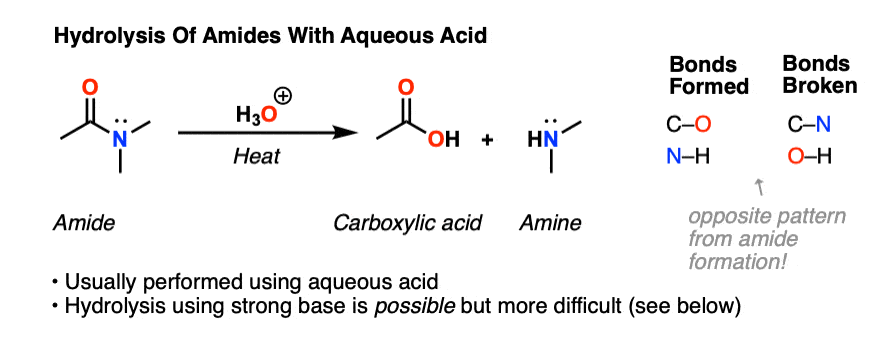
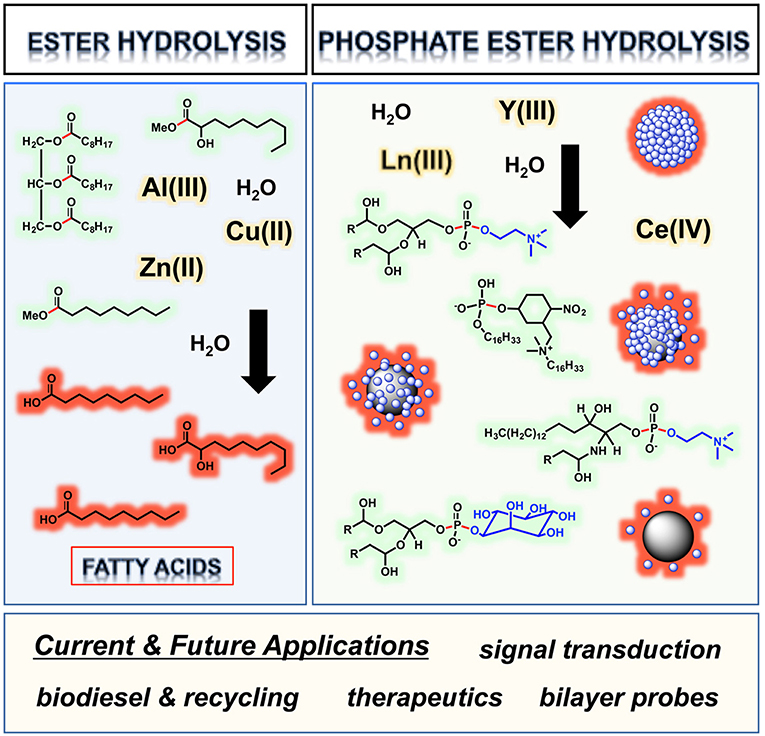
acid hydrolysis of metal complexes
|
Acid Hydrolysis of Co(III) amine complexes
The complexes of Co(III) containing N—H bonds undergo base hydrolysis often as much 106 times faster than the corresponding acid hydrolysis For example |
|
Reaction Mechanism in Transition Metal Complexes
Acid Hydrolysis B Mechanism of Acid Hydrolysis when the Inert Ligand is a pi Donor • It has been noticed that aquation of such trans complexes takes place |
What are the examples of acid hydrolysis?
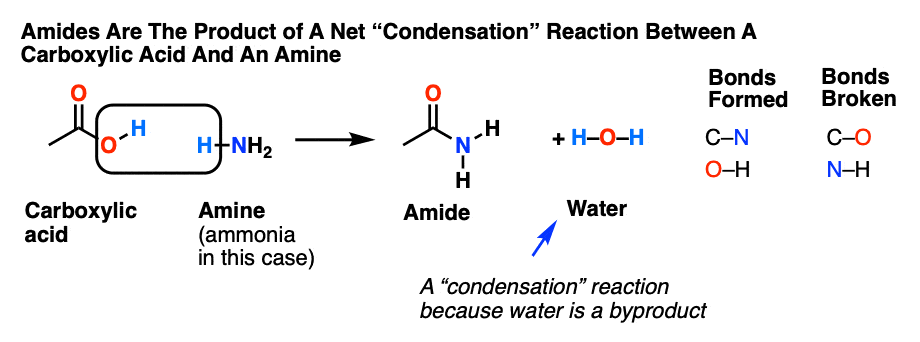
Acidic hydrolysis is simply the reverse of esterification.
The ester is heated with a large excess of water containing a strong-acid catalyst.
Like esterification, the reaction is reversible and does not go to completion.
As a specific example, butyl acetate and water react to form acetic acid and 1-butanol.Factors influencing dilute acid hydrolysis include the degree of grinding of the cellulosic feedstock, liquid-to-solid ratio, reaction temperature, reaction time, acid concentrations, and co-catalyst type and concentration.
What is acid hydrolysis in metal complexes?
Acid hydrolysis is a hydrolysis mechanism in organic chemistry in which protic acid is used to catalyse the cleavage of a chemical bond by means of a nucleophilic replacement reaction with the addition of water elements (H2O).
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo CHAPTER 3 Reaction Mechanism of Transition Metal Complexes – I:. |
|
BSc Chemistry
Metal-Ligand Equilibria and Reaction Mechanism of. Transition Metal Complexes). Module No. 25: Acid and Base hydrolysis Reactions without metal ligand bond |
|
M.Sc Semister I Paper I Inorganic Chemistry Department of
two types (a) when an aqua complex is formed by the replacement of a ligand by H2O molecules are called acid hydrolysis or equation reactions. |
|
HYDROLYSIS 2016.pdf
mechanisms account for neutral acid and base hydrolysis. The hydrolysis rates of halogenated aliphatic compounds ... of metal hydroxo complexes. |
|
Thioester Hydrolysis Reactivity of Metal Complexes
Scheme 1-4. Hydrolysis of adenosine triphosphate (ATP). In terms of hard/soft acid base theory the metal ions found in metallohydrolases are. |
|
Copper Complex Catalyzed Hydrolysis of Amides
in both acid and base catalyzed hydrolysis reactions. Metal ions were found to participate in enzyme mediated hydrolysis of esters and amides. |
|
Reaction Mechanism of Transition Metal Complexes – I
The thermodynamic stability of metal complexes is calculated by the overall Ligand Displacement Reactions in Octahedral Complexes- Acid Hydrolysis. |
|
Chemistry of macrocyclic complexes of cobalt (III). Acid hydrolysis of
metal also is bonded to four other ligands. It is ap- The acid hydrolysis of Co(t/WK[14]diene)C03 (where ?ra«s[14]diene is Curtis' Schiff base. |
|
Substitution reactions in octahedral complexes
Whereas the acid hydrolysis is occurred for reactions of metal complexes with water at pH < 3 base hydrolysis occurs in basic solutions (at pH > 10). |
|
Metal complex catalysis of the base hydrolysis of various amino acid
2514. Metal. Complex Catalysis of the Base Hydrolysis of Various. Amino Acid Esters Coordinated to the Complex of. Nitrilotriacetic Acid with. Copper (II). |
|
Reaction Mechanism in Transition Metal Complexes
Transition Metal Complexes ACID B Mechanism of Acid Hydrolysis when the inert ligand is a The rate of aquation of the following complexes follows the |
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Acid hydrolysis or aquation reactions may be defined as the reactions in which an aquo complex is formed due to the replacement of a ligand by water molecule Now, it has also been found experimentally that divalent monochloro complexes of Co(III) react at much slower than monovalent dichloro complexes |
|
BSc Chemistry - e-PG Pathshala
Metal-Ligand Equilibria and Reaction Mechanism of Transition Metal Complexes ) Module No 25: Acid and Base hydrolysis, Reactions without metal ligand |
|
Kinetics of the acid hydrolysis (aquation) - CORE
Elleman, Thomas Smith, "Kinetics of the acid hydrolysis (aquation) and isotopic exchange of choride with complexes represent an ideal class for ligand exchange studies tend to form double bonds with the central metal atom and that |
|
B Base Hydrolysis 25 - ScienceDirectcom
Octahedral Complexes A ACID HYDROLYSIS The most common reaction of a metal complex, and the one studied to the greatest extent, is the reaction |
|
RETROSPECTIVE ON STUDIES OF LIGAND - ScienceDirectcom
(b) Acid hydrolysis The first paper from our laboratory on the kinetics and mechanisms of substitution reactions of metal complexes was based on the work of |
|
MECHANISMS OF AQUATION AND BASE HYDROLYSIS IN
shown that when base hydrolysis of complexes of the types one or more substituents by water, the acid hydrolysis th metal ions such as TlJ+ and Ag+ |