acid hydrolysis vs base hydrolysis
Why base hydrolysis is faster than acid hydrolysis?
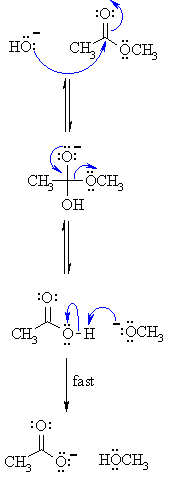
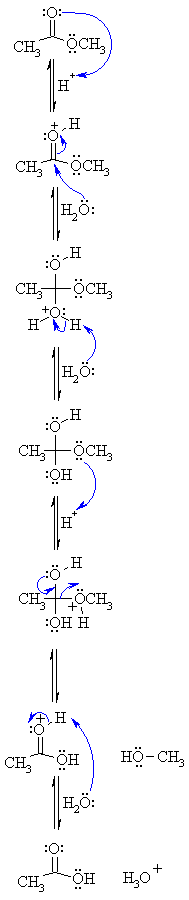
Acid catalyst provides H+ ions that accelerate the rate of hydrolysis by withdrawing electron density of the atom bearing the leaving group, thus making it more susceptible to nucleophilic attack by H20, while base catalyst provides OH− ions that are much stronger nucleophile than water, and hence accelerates the rate
What is a base hydrolysis?
And from there, we learned that basic hydrolysis is when water acts as an acid to break apart a weak base.
For example, water can react with urea, forming a hydroxide anion, which can then release ammonia from the urea, which is critical for the growth of plants.15 mar. 2022Why is alkaline hydrolysis better than acid hydrolysis?
Hydrolysis using dilute alkali
There are two big advantages of doing this rather than using a dilute acid.
The reactions are one-way rather than reversible, and the products are easier to separate.
|
Acid and Alkaline Hydrolysis Extraction of Non-Extractable
from blueberries with acid or alkaline hydrolysis methods and the related extraction conditions were (w/v) Na2CO3 solution were added |
|
Mechanism of Substitution Reactions in Complex Ions. VII. Base
Base Hydrolysis of Cobalt(III) Complex Ions rule the relative rates of base hydrolysis and acid ... By interpolation from a plot of log k versus. |
|
HYDROLYSIS 2016.pdf
Hydrolysis reactions are generally enhanced by both acids and bases and three independent reaction mechanisms account for neutral acid and base hydrolysis. |
|
Of the Base Hydrolysis of Various Amino Acid Esters Coordinated to
comparison of the rates of base hydrolysis of the ethyl esters of -amino acids H2NCHRCÓ2Et |
|
Dilute-acid Hydrolysis of Cellulose to Glucose from Sugarcane
w/v) reaction time (10-30 min) and incubation temperature (155-175 ºC). bases. Acid hydrolysis is an old process and in which the residual acid process ... |
|
Kinetics and Mechanism of Base Hydrolysis of (Dimethyl Sulphoxide
for the base hydrolysis and the first-order rate constants of the acid hydrolysis Table V shows that the rate acceleration for the N03- leaving ligand. |
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Base hydrolysis reactions may be defined as the reactions in which a hydroxo complex is formed due to the replacement of a ligand by hydroxyl ion. Base |
|
Critical review of hydrolysis of organic compounds in water under
15 oct. 2009 typical log kh vs pH plot for compounds which undergo acid . water |
|
The Composition and Antioxidant Activity of Bound Phenolics in
7 déc. 2020 by acid hydrolysis alkaline hydrolysis |
|
A new meaning of the terms acid and base hydrolysis
the acid-base properties of the solution) the term “acid hydrolysis” is confusing. Therefore forthis reaction we propose the term “aqualysis. |
|
HYDROLYSIS
It is generally observed that base catalyzed hydrolysis favours P-O or P-S cleavage, whereas neutral or acid catalyzed hydrolysis favours C-O or C-S cleavage |
|
Acid hydrolysis
Ester and amide bonds are particularly susceptible, and some ether-linked lipids can be saponified in acidic solution Basic hydrolysis (saponification) is more |
|
KINETICS AND MECHANISM OF ACID AND BASE HYDROLYSIS
Abstract-The kinetics of acid and base hydrolysis of chloro- and bromo-( cyclohexylamine)bis- (ethylenediamine) cobalt(Ill) complexes have been studied |
|
B Base Hydrolysis 25 - ScienceDirectcom
Thus substitution reactions are generalized acid-base reactions, where the such reactions are referred to as acid hydrolysis reactions, Kinetic studies |
|
Mechanisms of Lactone Hydrolysis in Acidic Conditions
3 jui 2013 · 1 INTRODUCTION As is also the case with neutral and base-catalyzed mechanisms, the acid-catalyzed hydrolysis of esters has seldom been |
|
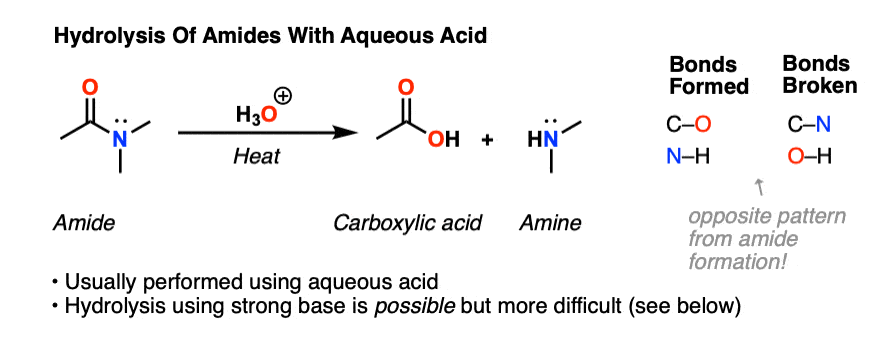
Lecture 6: Hydrolysis Reactions of Esters and Amides
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions form new esters by base- or acid-catalysed transesterification mechanisms; |