acidity of carboxylic acid and alcohol
|
Chapter 5 Carboxylic Acids and Esters
Esters may be broken apart under acidic conditions by water (a hydrolysis reaction) to form a carboxylic acid and an alcohol • This is essentially the |
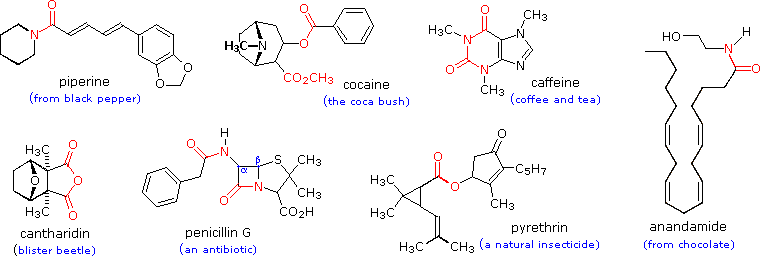
How does the acidity of a carboxylic acid compare to that of an alcohol?
The acidity of a carboxylic acid is higher than alcohol and even phenols.
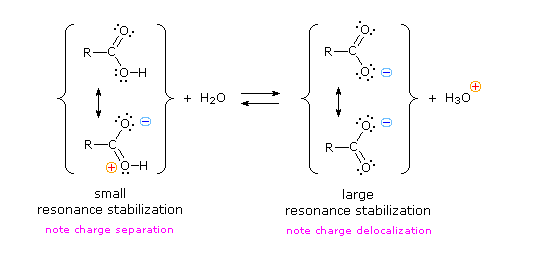
As discussed above, the carboxylate ion, the conjugate base of a carboxylic acid is stabilized by two equivalent resonance structures in which the negative charge is effectively delocalized between two more electronegative oxygen atoms.4 déc. 2019Why are the OH groups of carboxylic acids more acidic than alcohols?
The common explanation for why carboxylic acids are more acidic than other molecules (such as alcohols) is that resonance delocalization of charge stabilizes the conjugate base anion relative to the reactant acid.
Which is more acidic alcohol or carboxylic acid?
Resonance stabilization of their conjugate base.
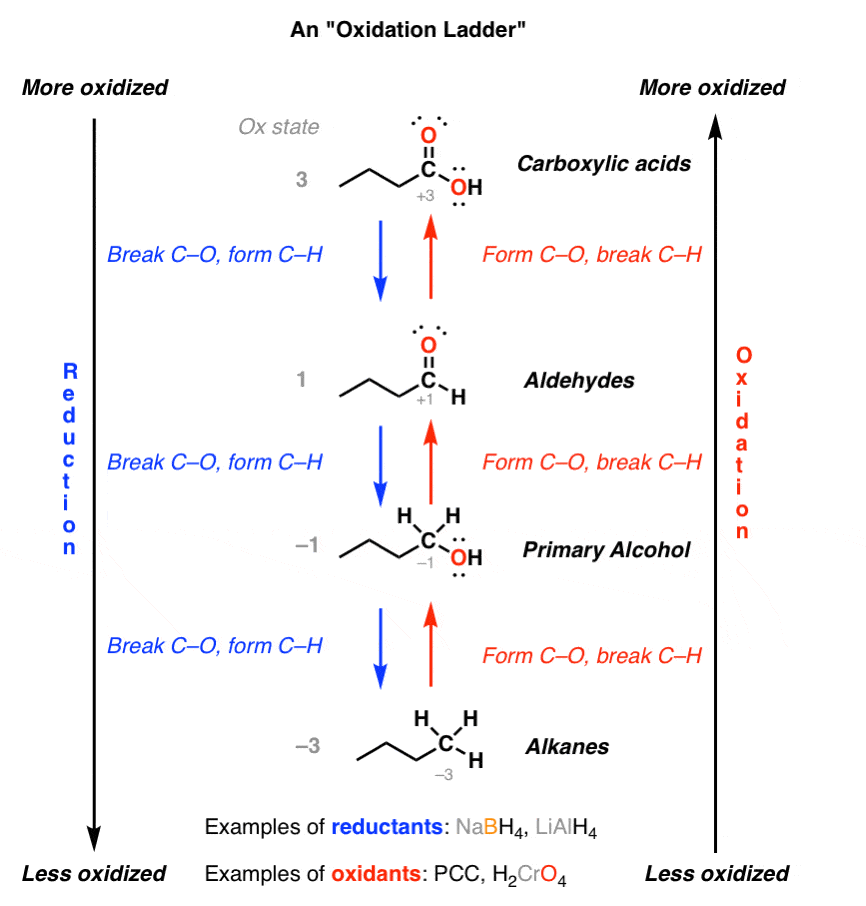
The resonance effect described here is undoubtedly the major contributor to the exceptional acidity of carboxylic acids.
However, inductive effects also play a role.
For example, alcohols have pKa's of 16 or greater but their acidity is increased by electron withdrawing substituents on the alkyl group.30 mai 2020
|
Chapter 5 Carboxylic Acids and Esters
Esters may be broken apart under acidic conditions by water (a hydrolysis reaction) to form a carboxylic acid and an alcohol. • This is essentially the |
|
Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
explain the mechanism of a few selected reactions of aldehydes and ketones;. • understand various factors affecting the acidity of carboxylic acids and their |
|
The role of resonance and inductive effects in the acidity of
Vinyl alcohol and vinyl alkoxide are included in the population analysis for comparison. The greater acidity of carboxylic acids compared to alcohols. |
|
Acidity of Carboxylic Acids: Resonance Delocalization or Induction?
On the basis of calculated acidities of the vinylogues of formic acid and vinyl alcohol Dewar and. Krull have concluded that the higher acidity of |
|
Role of .pi.-Electron Delocalization in the Enhanced Acidity of
acid vinyl alcohol |
|
Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
explain the mechanism of a few selected reactions of aldehydes and ketones;. • understand various factors affecting the acidity of carboxylic acids and their |
|
Carboxylic Acid Structure and Chemistry: Part 2
As a result of their relatively acidic nature carboxylic acids will ionize reaction and the role of the acid and alcohol nucleophile are illustrated in ... |
|
Information Sheet for Teachers: St. Elmo Brady (1884 - 1966) This
Derick and the Harvard chemist Arthur Michael disagreed on how the acidity of carboxylic acids was affected by replacing hydrogen atoms on the carbon chain with |
|
Origin of the Acidity of Enols and Carboxylic Acids
Abstract: The origin of the acidity of carboxylic acids and enols has been examined vinyl alcohol to its alkoxide ion the ? electrons of the proton are ... |
|
Carboxylic Acid Structure and Chemistry
Carboxylic acids are referred to as "weak acids" because they partially dissociate in water conjugate base formed from carboxylic acids (where the charge is delocalized by resonance), it is less likely to form Thus alcohols are less acidic than carboxylic acids |
|
Chapter 18: Carboxylic Acids 181: Carboxylic Acid Nomenclature
They are significantly more acidic than water or alcohols Bronsted Acidity (Ch 1 13): Carboxylic acids transfer a proton to water to give H3O+ and carboxylate |
|
Carboxylic Acids
alcohols (pKa 16 18) because resonance stabilizes the carboxylate anion by tivity than carbon increases the acidity of carboxylic acids, often by several orders |
|
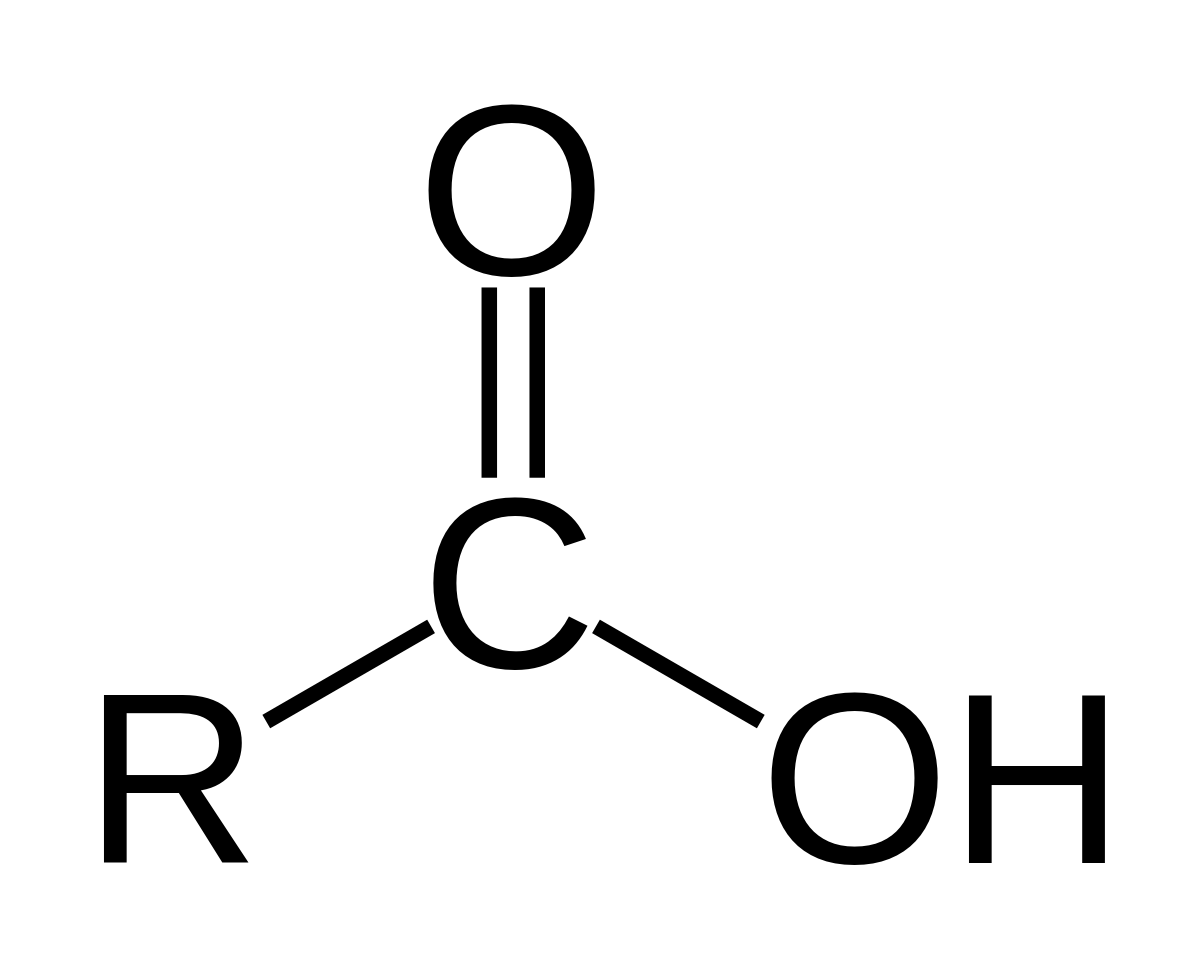
CARBOXYLIC ACIDS
Carboxylic acids are organic compounds containing the carboxyl group (-COOH), wherein the alcohols with acidic chromic acid solutions usually forms esters |
|
Carboxylic acids and their derivatives - Caltech Authors
Thus the K, of ethanoic acid, CH,C02H, is 1011 times larger than that of ethanol, CH3CH20H The acidity of the carboxyl group arises, at least in part, from the |
|
Nomenclature of Carboxylic Acids - Angelo State University
Learn the IUPAC system for naming carboxylic acids and esters Alkanes Water Solubility: Carboxylic acid Alcohols Aldehydes/Ketones Ethers Alkanes (low pH) carboxylic acid carboxylate ion 33 Carboxylate Salts Carboxylate Salts |
|
Carboxylic Acids A carbonyl with one OH attached is called a
Acidity As observed previously, carboxylic acids are far more acidic than alcohols This is due to the stability of the anion formed after deprotonation |
|
Aldehydes, Ketones and Carboxylic Acids - NCERT
In fact, carboxylic acids are amongst the most acidic organic compounds you have studied so far You already know why phenols are more acidic than alcohols |
|
Carboxylic Acids - De Boeck Supérieur
Esters, which are derived from the reaction of carboxylic acids and alcohols, are responsible for the Ka = 1 8 × 10–5 M The acidic nature of carboxylic acids |








![Alcohol Reactions [Reaction Map PDF] – Master Organic Chemistry Alcohol Reactions [Reaction Map PDF] – Master Organic Chemistry](https://image.slidesharecdn.com/carboxylicacid-190311055051/95/carboxylic-acid-6-638.jpg?cb\u003d1552616244)