activation energy of hydrolysis of ethyl acetate
Answer: c Explanation: The equilibrium position of ethyl acetate hydrolysis was demonstrated to be temperature independent.
Because the bonds broken and created are of the same kind, calculating the heat of reaction using the bond energies technique yields a value of zero.
What is the rate of hydrolysis of ethyl acetate?
Conclusion: In conclusion, it can be seen from this experiment that rate of reaction is concentration dependent while rate constant is not.
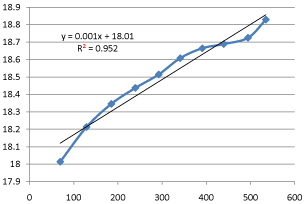
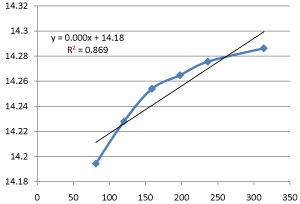
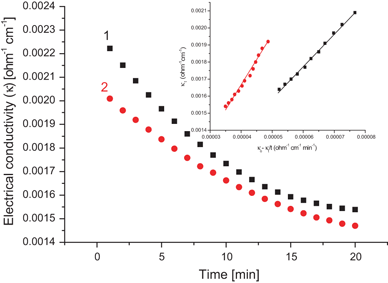
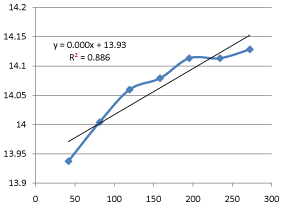
Also, that the rate constant for the hydrolysis of ethyl acetate with sodium hydroxide using HCl as a catalyst at 28oC is approximately 0.003min-1cm-3.
What is the activation energy of saponification of ethyl acetate?
In their study, they used 0.
8) M ethyl acetate and 1.
0) M NaOH and conducted the experiments at five different temperatures ranging form 30 to 70°C.
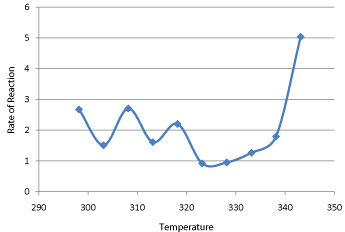
They obtained the value of activation energy, 46.5 kJ mole−1, which was about 10% higher than the present value.
What is the reaction of the hydrolysis of ethyl acetate ester?
(C) Pseudo first order reaction.
|
The Reaction Rate of the Alkaline Hydrolysis of Ethyl Acetate
and the activation energy was 11.56 kcal./mol. values agreed well with those of previous studies. From the standpoint of the electronic theory of organic |
|
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
frequency factor 27038. Keywords: Activation energy arrhenius equation |
|
Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
Chemical reactions reaction rate. Chemical kinetics is the part of physical chemistry that studies reaction rates. The reaction. |
|
Reaction Rate of the Alkaline Hydrolysis of Ethyl Acetate
The difference in the activation energies of the forward and reverse reaction rates was calculated from the experi- mental data. At lower temperatures this |
|
Kinetic study of hydrolysis of ethyl acetate using caustic soda
Chemical kinetics is the part of physical chemistry that studies reaction rates of the reaction. As in this project we study the kinet- ics of saponification |
|
Acid-catalyzed Hydrolysis of Ethyl Acetate
energy of activation gives 4.55 X 10-s. In their experiments the ester concentration was. 0.05 M and the medium contained about 1.5 ml. more water per 100 ml |
|
Temperature Dependence of Ester Hydrolysis in Water - Gunnar
The activation energy EA |
|
PRESSURE EFFECT AND MECHANISM IN ACID CATALYSIS: VII
The volumes of activation for the acid-catalyzed hydrolysis of illethyl and ethyl Thus for the hydrolysis of ethyl acetate in dioxane-water mixtures the ... |
|
DFT and CBS Study of Ethyl Acetate Conformers in the Neutral
transition state conformational effect on the activation energy of ethyl acetate neutral hydrolysis. Heliyon 5: e02409. Fei Z. |
|
Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
1 de fev. de 2017 whereas ethanol as a by-product can be used as a bio fuel. Despite the. Research Article. Kinetics of Alkaline Hydrolysis of Ethyl Acetate by ... |
|
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
frequency factor 27038. Keywords: Activation energy arrhenius equation |
|
The Reaction Rate of the Alkaline Hydrolysis of Ethyl Acetate
The difference in the activation energies of the forward and reverse reaction rates was calculated from the experi- mental data. At lower temperatures this |
|
IV SEMMESTER
KINETICS OF ACID HYDROLYSIS OF AN ESTER. AIM: To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as a catalyst. PRINCIPLE:. |
|
Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
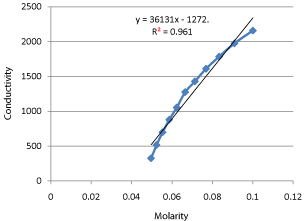
01-Feb-2017 Kinetics of Alkaline Hydrolysis of. Ethyl Acetate by Conductometric Measurement Approach Over Temperature Ranges (298.15-343.15K). Austin. |
|
Kinetics of the Saponification of the Ethyl Esters of Normal Aliphatic
recrystallized from hot ethanol melted at 121-122°; ing point determination of its nienthyl ester. ... Kinetics of Hydrolysis of Some Ethyl «-Esters. |
|
Temperature Dependence of Ester Hydrolysis in Water - Gunnar
The rate of the hydroxyl-promoted hydrolysis of ethyl acetate has been Arrhenius activation energy of the alkaline and acid hydrolysis shows an ... |
|
Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
Chemical reactions reaction rate. Chemical kinetics is the part of physical chemistry that studies reaction rates. The reaction. |
|
Effect of Ion Exchange Resin Catalyst on Hydrolysis of Ethyl Acetate
Hydrolysis reaction of ethyl acetate is a reversible reaction with high activation energy. The reaction has a very slow reaction rate when carried out in |
|
Reaction Rate of the Alkaline Hydrolysis of Ethyl Acetate
of the ester and the base were close together. The initial rate constant at 25°C was measured as 0.1120 1./mol./sec. and the activation energy was 11.56 |
|
Kinetic study of hydrolysis of ethyl acetate using caustic soda
Chemical kinetics is the part of physical chemistry that studies reaction rates of the reaction. As in this project we study the kinet- ics of saponification |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 nov 2015 · Keywords: Saponification, arrhenius equation, activation energy, rate constant Introduction Saponification reaction is the hydrolysis of a |
|
Exercise 8 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
1 Exercise 8 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE Theory CHEMICAL KINETICS Chemical reactions, reaction rate Chemical kinetics is the |
|
KINETICS OF HYDROLYSIS OF ETHYL ACETATE
KINETICS OF HYDROLYSIS OF ETHYL ACETATE 1 The kinetics data will be obtained From these data, the order of the reaction, the rate constant and the |
|
Reaction rate and activation energy of the acidolysis - PHYWE System
30 mar 2017 · Experiment: Reaction rate and activation energy of the acidolysis of ethyl acetate The acid ester hydrolysis is described by the equilibrium |
|
Hydrolysis of Ethyl Acetate
where a is the initial molarity of ethyl acetate, b is the initial molarity of sodium hydroxide, x is the amount of reactant lost in time t, and k is the rate constant The |
|
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
ABSTRACT Hydrolysis is a chemical decomposition involving breaking of a bond and the addition of elements of water In this hydrolysis of ester (ethyl acetate) |

![PDF] Catalytic Hydrolysis of Ethyl Acetate using Cation Exchange PDF] Catalytic Hydrolysis of Ethyl Acetate using Cation Exchange](https://i1.rgstatic.net/publication/318636753_Shifting_Order_kinetic_Study_for_Alkaline_Hydrolysis_of_Ethyl_Acetate/links/597321e8458515e26dfdaf10/largepreview.png)