activation energy of saponification of ethyl acetate
|
Experimental Studies of Ethyl Acetate Saponification Using Different
4 fév 2019 · Table 4 Kinetic data of ethyl acetate saponification by sodium hydroxide Activation energy (kJ/mol) Pre-exponential factor |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 nov 2015 · The aim of this scientific research is to estimate the parameters of Arrhenius equation which are rate constant and activation energy for ethyl |
What is the saponification reaction of ethyl acetate?
The hydrolysis of ethyl acetate with sodium hydroxide to produce ethanol and sodium acetate is known as a saponification reaction.
The end products of saponification reaction (i. e., sodium acetate and ethanol) are used in various fields – for instance, the petroleum, textile, cosmetics, and paint industries.For the saponification of ethyl acetate, the entropy of activation was determined to be ΔS‡ = −74.
7) J mol−1 K−1 and enthalpy of activation ΔH‡ = 46.3 kJ mol−1.
What is the activation energy of hydrolysis of ethyl acetate?
The initial rate constant at 25°C was measured as 0.1120 1./mol./sec. and the activation energy was 11.56 kcal./mol., values agreed well with those of previous studies.
What is the activation energy of the saponification reaction?
The determined activation energy of the Saponification reaction between olein and sodium hydroxide is 2.53 J mol−1.
However, there are a few interesting inferences that can be drawn from the results obtained.
|
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
For this reason in this study; it was determined the reaction order the forward rate constant |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 нояб. 2015 г. Keywords: Saponification arrhenius equation |
|
Experimental Studies of Ethyl Acetate Saponification Using Different
4 февр. 2019 г. Using the reaction rate constant at different temperatures the activation energy and the pre- exponential factor of ethyl acetate ... |
|
Kinetics of the Saponification of the Ethyl Esters of Normal Aliphatic
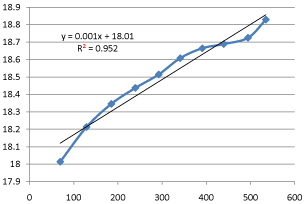
Saponification of Ethyl Acetate at 30° a = (Ester) = (NaOH) = 0.05 t min. X for by experimental error. Table III. Activation. Energies of. Saponification of. |
|
The enthalpy and entropy of activation for ethyl acetate saponification
24 янв. 2012 г. ABSTRACT: The saponification of ethyl acetate was measured by conductimetry at different temperatures within a batch reactor. |
|
Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
1 февр. 2017 г. Saponification of ethyl acetate with sodium hydroxide is the 2nd order overall 1st order with respect to reactants furthermore reaction order ... |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
constant and activation energy for ethyl acetate saponification. For this purpose the reaction is experimentally performed in a Batch Reactor and change in |
|
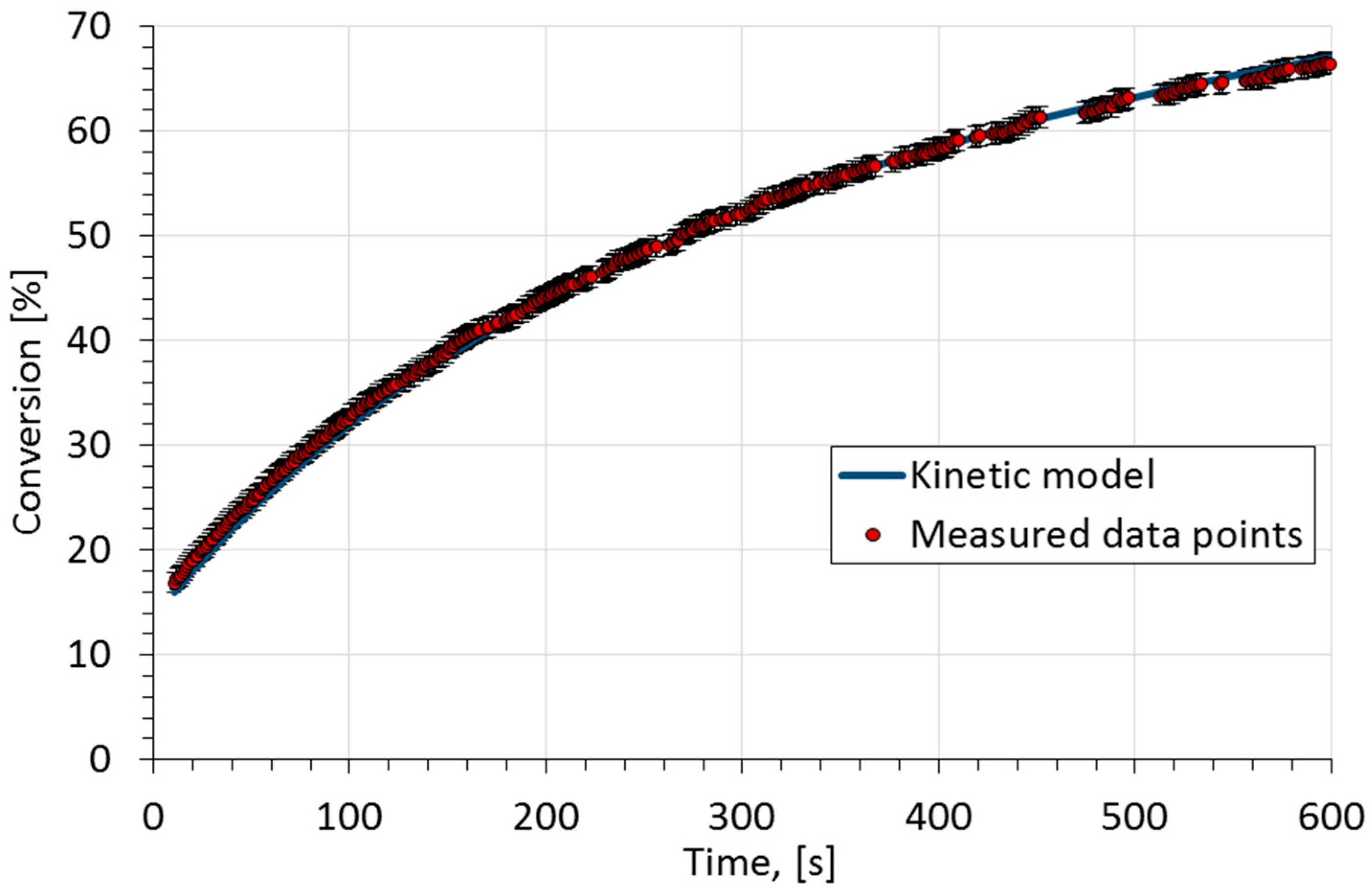
Kinetic studies on saponification of ethyl acetate using an innovative
on the rate constant and activation energy in the saponi- fication reaction of ethyl acetate and sodium hydroxide. [47–9]. While augmenting our new |
|
Estimation of Parameters of Kinetic Study and Arrhenius Equation
19 мар. 2019 г. Key Words: Ethyl Acetate Sodium Hydroxide |
| Aspen Plus Simulation of Saponification of Ethyl Acetate in the |
|
Experimental Studies of Ethyl Acetate Saponification Using Different
4 Feb 2019 Using the reaction rate constant at different temperatures the activation energy and the pre- exponential factor of ethyl acetate ... |
|
The enthalpy and entropy of activation for ethyl acetate saponification
24 Jan 2012 ABSTRACT: The saponification of ethyl acetate was measured by conductimetry at different temperatures within a batch reactor. |
|
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
Keywords: Activation energy arrhenius equation |
|
Estimation of Parameters of Kinetic Study and Arrhenius Equation
19 Mar 2019 reaction temperature. Key Words: Ethyl Acetate Sodium Hydroxide |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 Nov 2015 Keywords: Saponification arrhenius equation |
|
Kinetics of the Saponification of the Ethyl Esters of Normal Aliphatic
fluxed for eleven hours. Carbonation gave a 64% yield of o-ethoxybenzoic acid characterized by a mixed melt- ing point determination of its nienthyl ester. |
|
Saponification of Ethyl Acetate
dx dt. The expression e-AH/RT represents the fraction of molecules having an energy equal to or greater than the energy required for activation. For bimolecular |
|
Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
1 Feb 2017 Saponification of ethyl acetate with sodium hydroxide is the 2nd order overall 1st order with respect to reactants furthermore reaction order ... |
|
Kinetics of the Saponification of the Ethyl Esters of Several Phenyl
3. The steric influence of phenyl in the ex- position upon the rate and energy of activation for the saponification |
|
The Acid Catalyzed Hydrolysis of Phenyl Substituted Aliphatic Esters1
phenyl group into ethyl acetate is only 600 calories for saponification one would expect little change in the activation energy for theprocess of acid. |
|
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 nov 2015 · Keywords: Saponification, arrhenius equation, activation energy, rate constant Introduction Saponification reaction is the hydrolysis of a |
|
Saponification of Ethyl Acetate
Theory The rate constant k for chemical reactions is given by the Arrhenius equation: k = se-48a/RT where e = base of natural logarithms AH, = energy per |
|
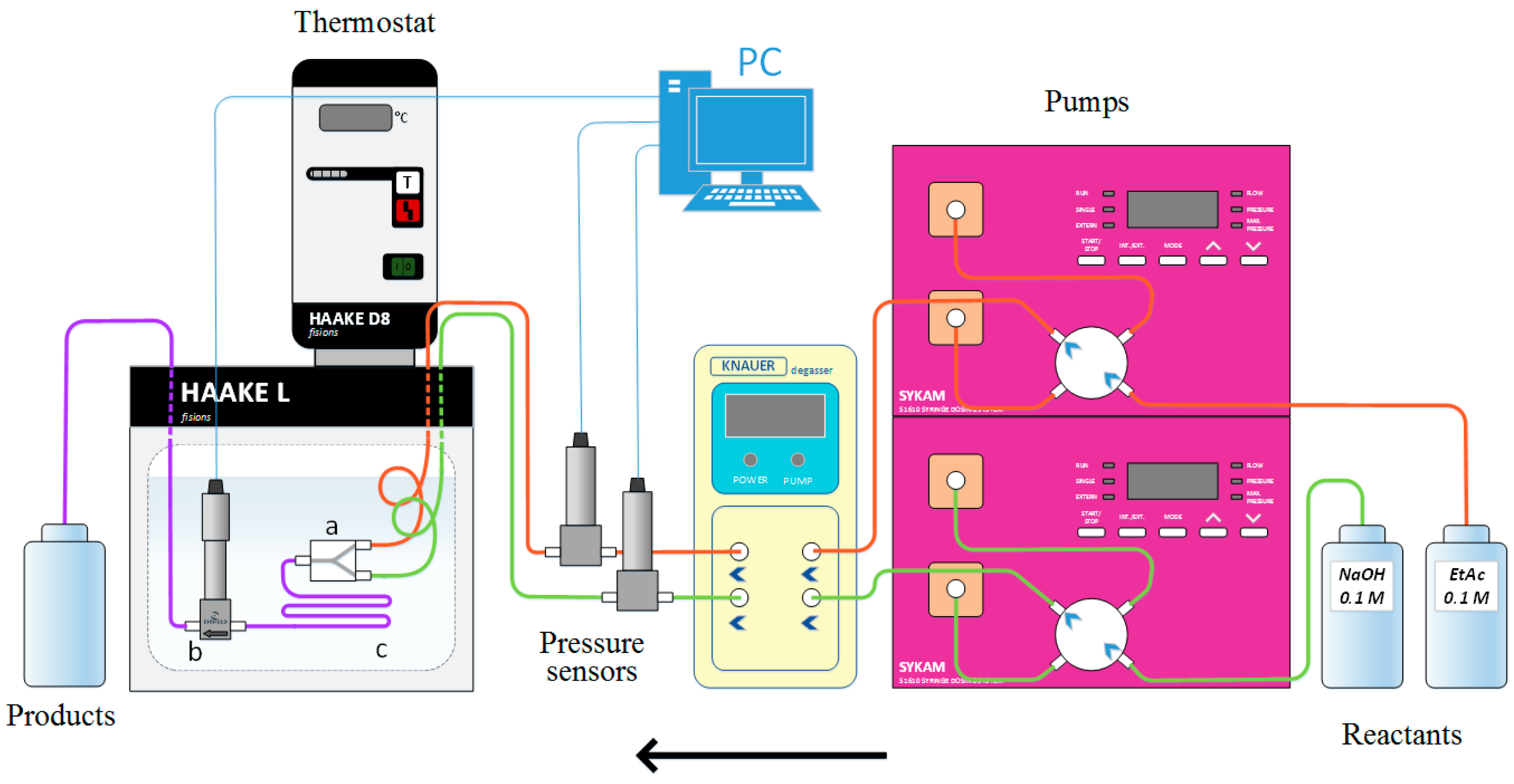
Aspen Plus® Simulation of Saponification of Ethyl Acetate in the
1 sept 2014 · The developed model can be used as a guide for understanding the reaction kinetics of a plug flow reactor Keywords: Aspen Plus; Modelling; |
|
Reaction Kinetics and Critical Phenomena: Saponification of Ethyl
The rate of sponification of ethyl acetate by sodium hydroxide was mea- sured near the consolute point of the liquid mixture, 2-butoxyethanol + water |
|
Kinetics of Alkaline Hydrolysis of Ethyl Acetate by Conductometric
1 fév 2017 · The average values of rate constant and activation energy are found Saponification of ethyl acetate with sodium hydroxide proceeds through |
|
Estimation of Parameters of Kinetic Study and Arrhenius - IRJET
19 mar 2019 · activation energy of reaction was calculated by Arrhenius equation, E= Kinetics of hydrolysis of ethyl acetate with sodium hydroxide has been |