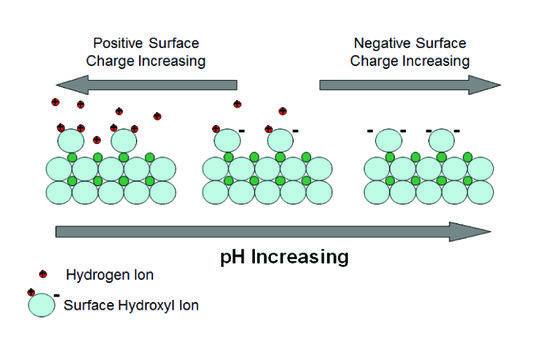
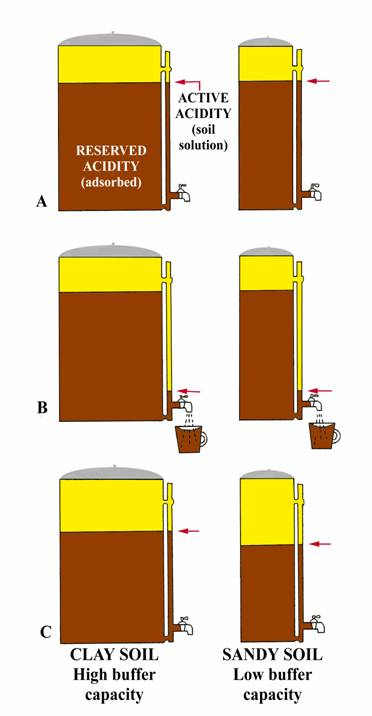
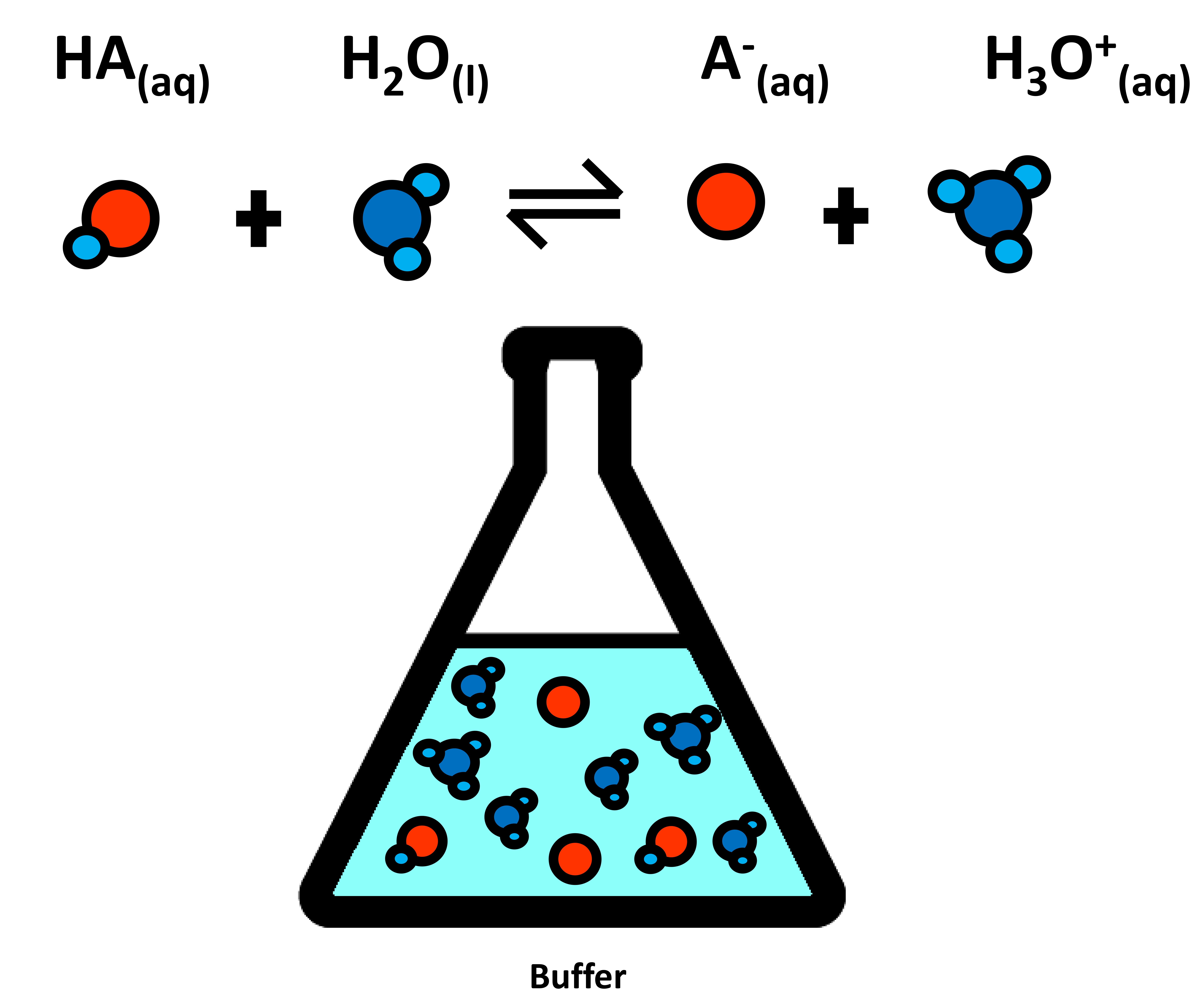
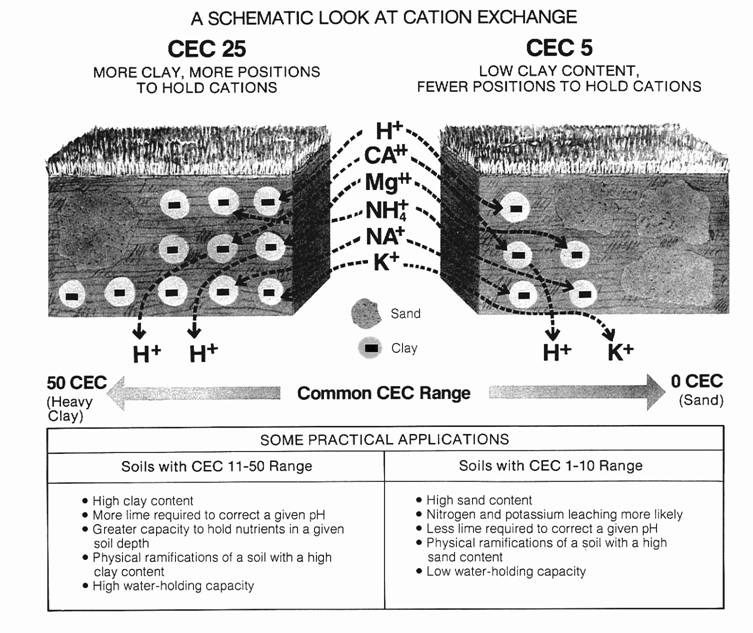
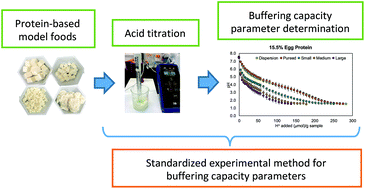
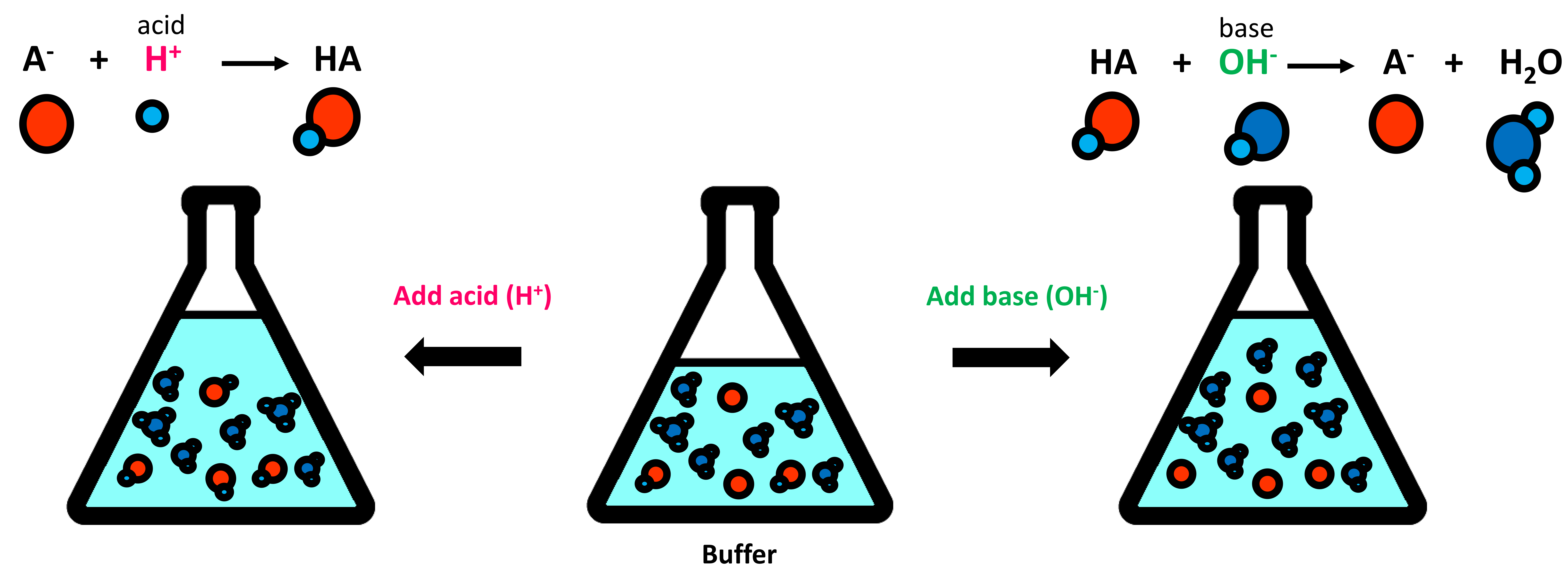
buffer capacity definition
Buffer capacity is higher, when the components of a buffer are in higher concentration.
For instance, a 2.
0) M buffer concentration has a greater buffer capacity than a buffer with a concentration of 0.
1) M.
Buffer range is the effective range of pH which is used for the determination of the working pH range of buffer.
What is the buffer capacity rule?
The buffer capacity is defined as the amount of acid or base you can add without changing the pH by more than 1 pH unit.
I will define "significant change" as 1 pH unit. ∴ We can add 0.294 mol of base before the pH changes by 1 unit.
What is meant by buffer capacity?
Buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its pH to change by 1 unit.24 août 2021
What is pKa and buffer capacity?
The pKa of a buffer is commonly perceived as the pH of the said buffer when the concentrations of the two buffering species are equal, and where the maximum buffering capacity is achieved.
However, it is often forgotten, that when defined as above, pKa depends on buffer concentration and temperature.
|
An Algebraic Derivation of Buffer Capacity
specialized topics such as buffers and their capacities. Currently introductory chemistry students struggle to fully understand the formal definition of buffer |
|
Research Article Numerical Computation of pH and Buffer Capacity
Since the charge balance is always defined in terms of equivalent unit the negative and positive species are referred to basic and acidic compounds |
|
ทฤษฎีของปฏิกิริยากรด – เบส
จงบอกความหมายของสารละลายบัฟเฟอร์ (Buffer Solution) และบัฟเฟอร์คาพาซิตี (Buffer capacity) 1. นิยามของกรด-เบส (Definition of acids and bases). ทฤษฎีที่อธิบาย ... |
|
Buffer Index and Buffer Capacity for a Simple Buffer Solution
where C is the initial concentration of electrolyte solution. According to IUPAC (6) the definition of buffer index or buffer capacity is as follows: “The |
|
Buffering Capacity in Human Skeletal Muscle: A Brief Review
curve which means the number of mmoles of H+ required to change the pH of. 1 Sahlin K |
|
Buffer Index and Buffer Capacity for a Simple Buffer Solution
where C is the initial concentration of electrolyte solution. According to IUPAC (6) the definition of buffer index or buffer capacity is as follows: “The |
|
Availability of Soil Potassium to Ryegrass: 2. Potassium Buffering
fertilised soils. RESULTS AND DISCUSSION. Definition of K buffering capacity. The Q/I method is used to obtain the adsorption isotherm between K and Ca(Mg) of |
|
UNDERSTENDING THE BUFFERING CAPACITY IN FEEDSTUFFS1
Key words: pH value feedstuffs |
|
2018 LIST OF SELECTED BUFFER CAPACITY PROJECTS
use of a buffer capacity composed by two aerial means that will top-up the existing response resources made available by Italy as part of its national |
|
Integrated Optimization of Mixed-Model Assembly Line Balancing
precedence constraint to define the buffers capacity among stations |
|
Moisture Buffer Capacity of Different Insulation Materials
Moisture buffering capacity was defined as a weight change of the sample as a function of the ambient RH change and measured for wood brick |
|
Buffer capacity: capturing a dimension of resilience to climate
5 Jan 2013 having buffer capacity means having the capacity to cushion change and possibly to use the emerging opportunities to. |
|
Determination and Analysis of the Buffer Capacity of Isolated
The ability of isolated chloroplasts to bind protons has been measured by means of titration with acid or alkali over the range ofpH 3.7 to 10.8. |
|
Buffer Index and Buffer Capacity for a Simple Buffer Solution
where C is the initial concentration of electrolyte solution. According to IUPAC (6) the definition of buffer index or buffer capacity is as follows: “The |
|
Preliminary findings on the correlation of saliva pH buffering
27 Jan 2017 were correct. Salivary buffering capacity is defined as the ability of saliva to buffer acids produced by bac-. |
|
Characterization of proteins by means of their buffer capacity
Keywords: coulometry ISFET |
|
An Algebraic Derivation of Buffer Capacity - World Journal of
buffer capacity definition to the following: Definition 1 The buffer capacity of a solution is the amount (in mol) of strong acid or strong base that must be. |
|
JCE0897 p937 Buffer Index and Buffer Capacity for a Simple Buffer
where C is the initial concentration of electrolyte solution. According to IUPAC (6) the definition of buffer index or buffer capacity is as follows: “The |
|
A model of sizing production buffer stocks based on the processes
In order to define the optimal buffers the authors applied theory of constraints Internal efficiency: is the capacity to be efficient during operational ... |
|
An Algebraic Derivation of Buffer Capacity - World Journal of
buffer capacity definition to the following: Definition 1 The buffer capacity of a solution is the amount (in mol) of strong acid or strong base that must be added to one liter of solution to change either the pH or pOH by one unit |
|
Buffering Capacity in Human Skeletal Muscle: A Brief Review - CORE
This is defined as buffering capacity, which is a potent protective mechanism to prevent pH decrement from excessive acidosis Thus high buffering capacity in |
|
PH buffers Buffer capacity and buffering range
Buffer capacity and buffering range 1 The term "buffer capacity" (β) quantifies the change in pH of the solution moment, defining the first calibration point |
|
A buffer is a solution that resists changes in pH upon the addition of
State the operational and technical definitions of a buffer Buffering capacity refers to the amount of added acid or added base that can be neutralized by a |
|
Buffer Capacity
Quantitative measure of this resistance to pH changes is called buffer capacity ▫ Buffer capacity can be defined as the number of moles of H+/OH- ions that |
|
Characterization of proteins by means of their buffer capacity
It will be shown that the buffer capacity can be measured with an ISFET- based sensor-actuator device The alternating generation of protons and hydroxyl ions by |
|
Buffering Capacity - ScienceDirectcom
BUFFER CAPACITY AND BUFFER RESOURCE According to definition [1-3], the buffer capacity is the derivative dCb fl = (8 1) d(pH) where Cb represents the |