buffer capacity experiment procedure
|
Buffer Capacity
In this experiment you will prepare several different buffer solutions and determine the buffer capacity for each Part of the data analysis will include a |
|
Lab 4: Designing and Preparing a Buffer Bellevue College
Select one buffer system to work with for part 2 of this experiment that you can prepare from two of Compare this to the volume of conjugate base solution |
|
Experiment 12 Buffer solutions 1
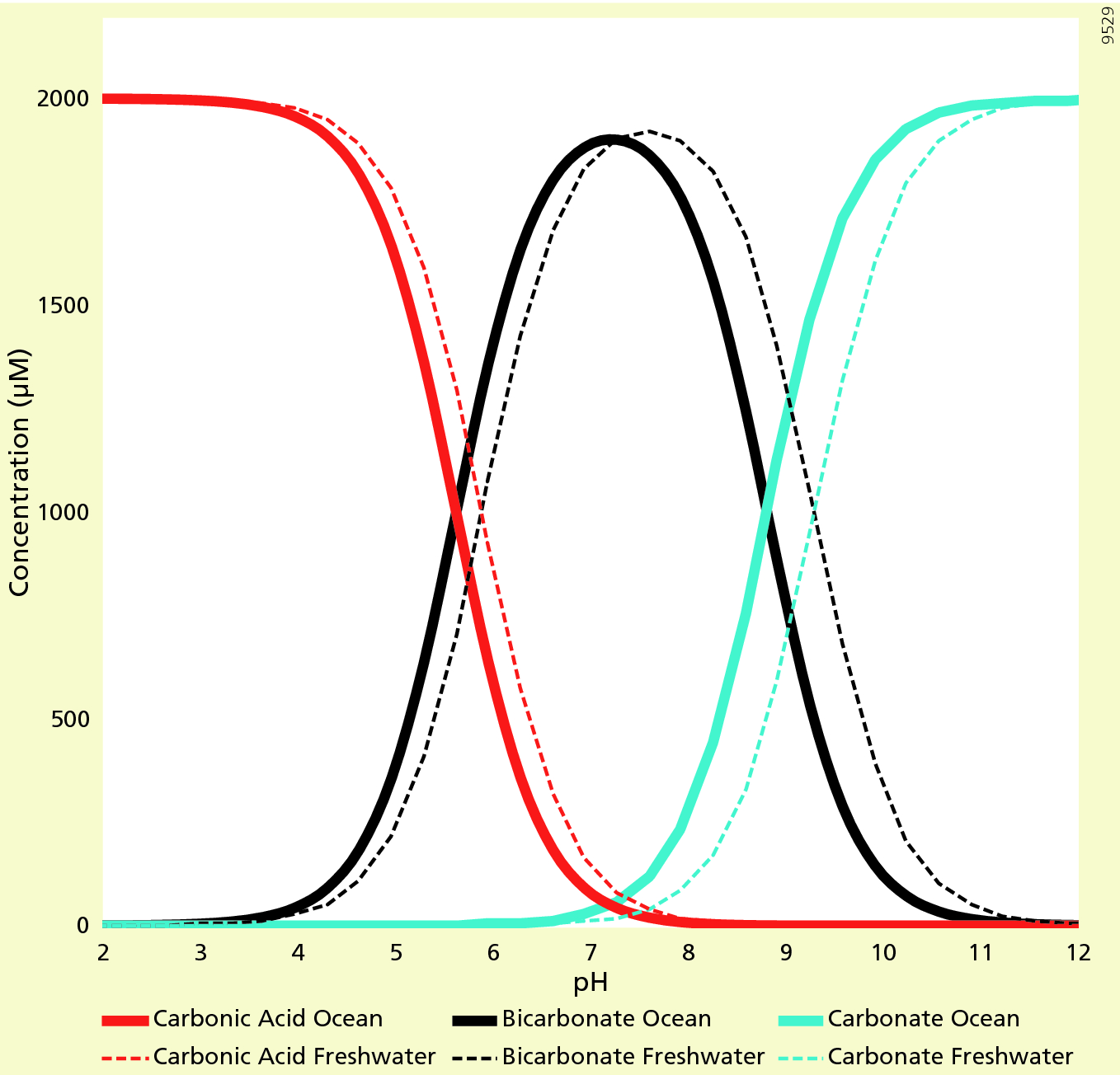
A buffer solution has the greatest capacity when the concentration ratio of the two components is 1 and better within one pH unit of pKa In this experiment |
|
7—Investigation of Buffer Systems
Buffer capacity is a quantitative measure of the resistance of a buffer solution to change in pH on addition of strong acid or base Normally it can be defined |
|
Experiment-11-Chem-1Bpdf
If your buffer is basic calculate the volume (in mL) of 0 0010 M NaOH needed to prepare 100 mL of a solution that has the same pH as your buffer Procedure |
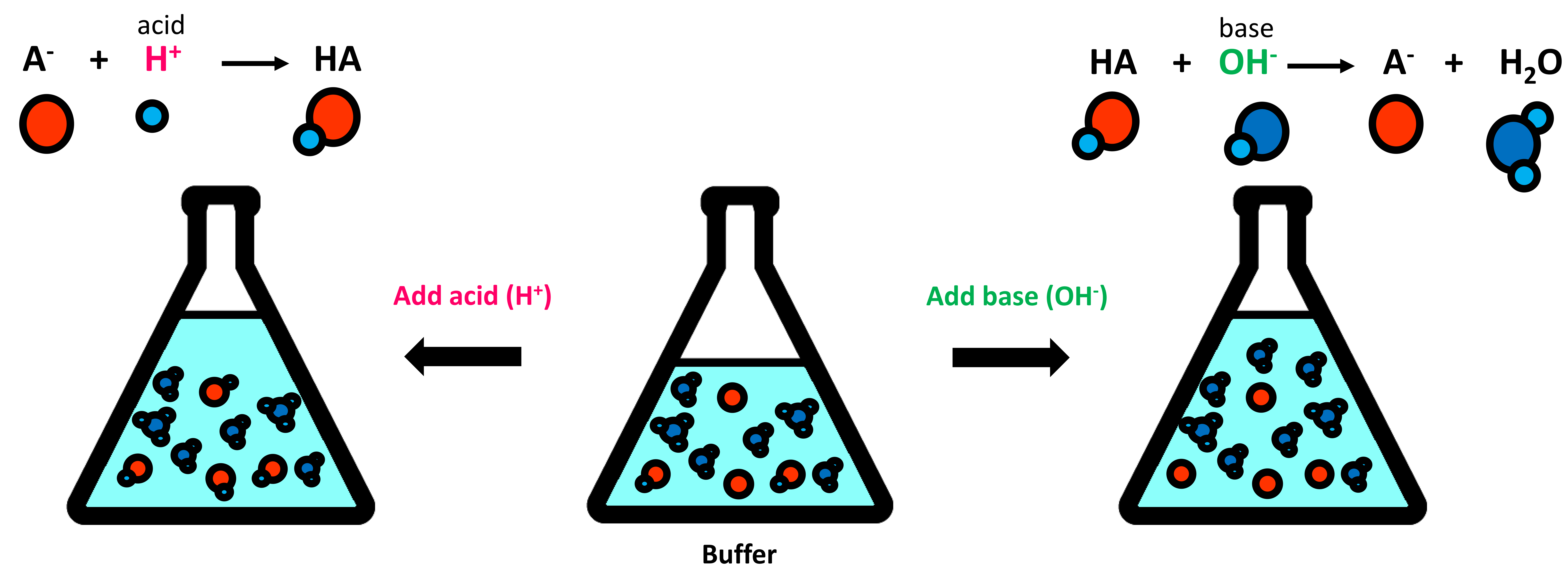
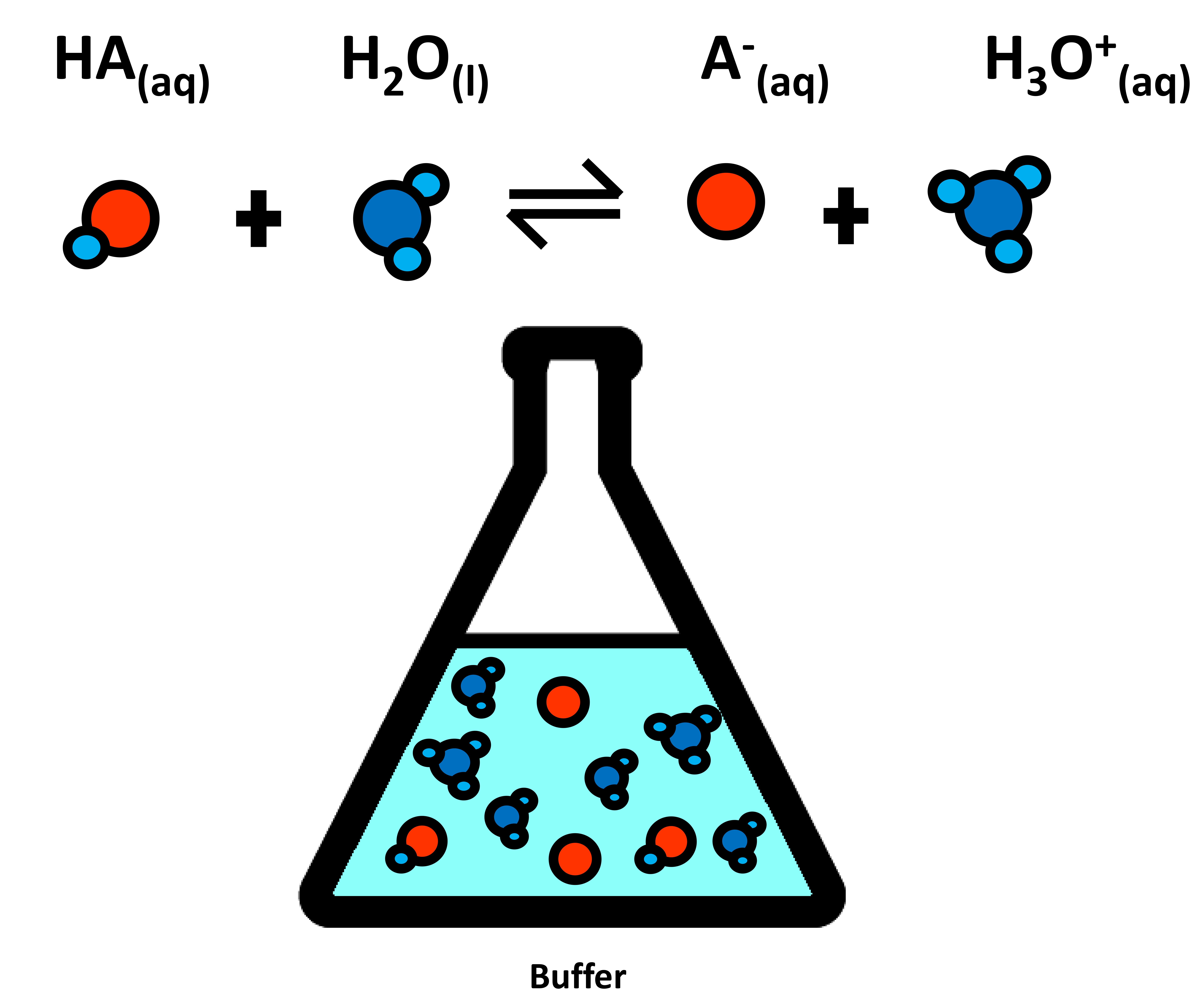
Buffer capacity is a quantitative measure of the resistance of a buffer solution to change in pH on addition of strong acid or base.
Normally it can be defined as the millimoles of strong acid or base required to change the pH of 1 liter of buffer by ±1.0 unit.
What is the procedure for making a buffer?
Preparing buffers consists of several steps: Calculate the components (concentrations and amounts) according to the required use and target volume, weigh-in the components, dissolve the components, adjust the pH, fill-up to the final volume, label, document results and use directly or store for later usage.
How do you experimentally determine buffer capacity?
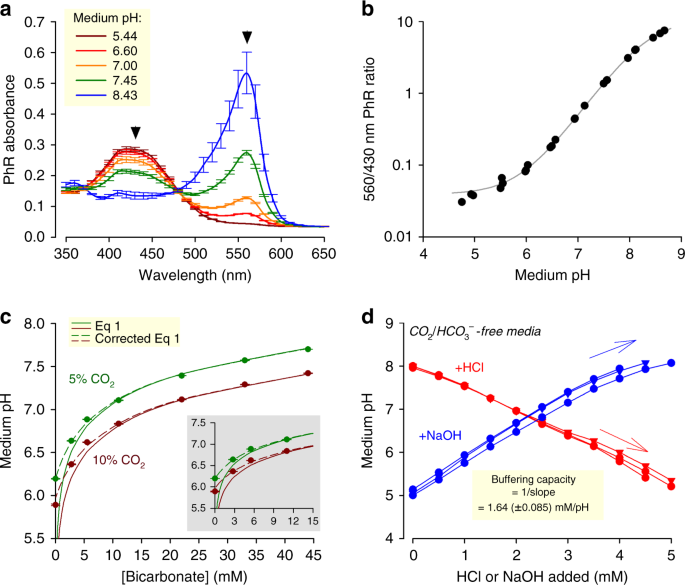
Buffer capacity is determined through a titration, a technique in which a known volume and concentration of a base or acid is added to the analyte of unknown concentration (Figure 2).
What is the method of buffer capacity test?
Buffer capacity is determined by quantitative test using a hand-held pH meter method [Figure 2].
This method involves the addition of 0.5 ml of saliva to 1.5 ml of 5 mmol/L Hcl.
Mixture was vigorously shaken.
| Buffer Capacity |
|
Experiment 11 - Chem 1B
This solution will not be a buffer solution as can be shown by comparing its buffering ability to that of the buffer solution. Finally |
|
Buffer capacity: An undergraduate laboratory experiment
boiled water may also be used for making the solutions. Procedure. The procedure is straightforward inthat measurements of pH are made as a standard solution |
|
7—Investigation of Buffer Systems
Objectives. • Reinforce concepts of buffer buffer range and buffer capacity. • Learn how to prepare acid-base buffers. • Learn how to calculate the pH of a |
|
Experiment 7: Preparation of a Buffer
The preparation of buffer solutions is a common task in the lab will make 100 mL of a buffer also with pH = 5 |
|
Lime Requirement by Measurement of the Lime Buffer Capacity
The procedure for preparing saturated calcium hydroxide is available from the Soil Plant |
|
• Porosity of Compost • Water retention capacity of Compost
Experiment step by step: Procedure with Bunsen burner or hot plate . Experiment 4: Determination of the 'buffering capacity' . |
|
• Porosity of Compost • Water holding capacity of Compost • Organic
Experiment step by step: Procedure with Bunsen burner or hot plate . Experiment 4: Determination of the 'buffering capacity'. |
|
Experiment 32 BUFFERS1
3 janv. 2019 You must rely on your SC112 knowledge of buffers. Supplies on the submarine are limited and the only available pH meter is broken! Procedure: ... |
|
Lab. 6 - BUFFER SOLUtiONS
Lab. No. 6. Physical Pharmacy I. Buffer solutions Buffer capacity (?): is the quantity of strong acid or base that must be ... Procedure. 1. Buffer ... |
|
Buffer Capacity
Prepare buffer solutions At one of the burette stations, fill the left burette with 0 50M acetic acid and the right burette with 0 50M sodium acetate Using the burettes, add the volumes of acetic acid and sodium acetate given in Table 1 to 100-mL flasks Then, fill the flasks to the fiducial mark with DI water |
|
7—Investigation of Buffer Systems - James Madison University
Reinforce concepts of buffer, buffer range and buffer capacity Laboratory Notebook—prepared before lab (if require by your instructor) Procedure Part I-A: |
|
Experiment 32 BUFFERS1 - USNA
3 jan 2019 · Calculate the change in pH of a simple buffer solution of known composition ability of these molecules and ions to hold onto a proton in an Repeat the procedure in part 4a using 0 20 M NaOH (instead of 0 20 M HCl) |
|
1 Experiment 6: Buffers Reading: Sections 161-162 in Olmstead
Purpose: The buffering ability and properties under dilution of acetic acid- sodium acetate buffers will be determined A pH 5 or pH 9 buffer will be prepared using |
|
Experiment 7: Preparation of a Buffer - Plymouth State University
The preparation of buffer solutions is a common task in the lab, especially in you will make 100 mL of a buffer also with pH = 5, but with a higher buffering capacity, the needed calculations in the Procedure (Step B1) before coming to lab |
|
PH and Buffers Laboratory
buffering capacity In analyzing the bottom of the electrode with a lab wipe The electrode Place the electrode (using the procedures above) into a beaker of |
|
CHEM 113 GENERAL CHEMISTRY LABORATORY-I BOOKLET
EXPERIMENT 7: BUFFERS, BUFFER CAPACITY, AND BUFFERING ZONE and to know about the procedure of the regarding experiment and theoretical |
|
PREPARATION AND TESTING OF BUFFER SOLUTIONS
changes in pH The larger the buffer capacity, the greater the resistance to pH change part of the experiment into a large test tube Measure the If the results appear to be odd, it is a good idea to repeat the procedures in doubt Preparing a |
|
Buffer Preparation and Capacity
In lab this week you are going to prepare an assigned buffer solution and test the buffering capacity of the solution using strong acid (HCl) and strong base all calculations are complete, prepare a plan of procedure to prepare your buffer |
|
Lab 4: Designing and Preparing a Buffer - Bellevue College
Finally you will use equation (3) to design and prepare a buffer of a specific pH Procedure Part 1, pH of salt solutions: You will need a calibrated pH meter and 4 |