clinical trial protocol manuscript
|
Clinical study protocol template
Clinical Trial Protocol Template Version 1 0 (01-Dec-2017) This document (090095af8aea8d7f in docbase CREDI_EH) has been digitally signed with external signatures using Entrust PKI |
How can a study protocol be structured for randomised trials?
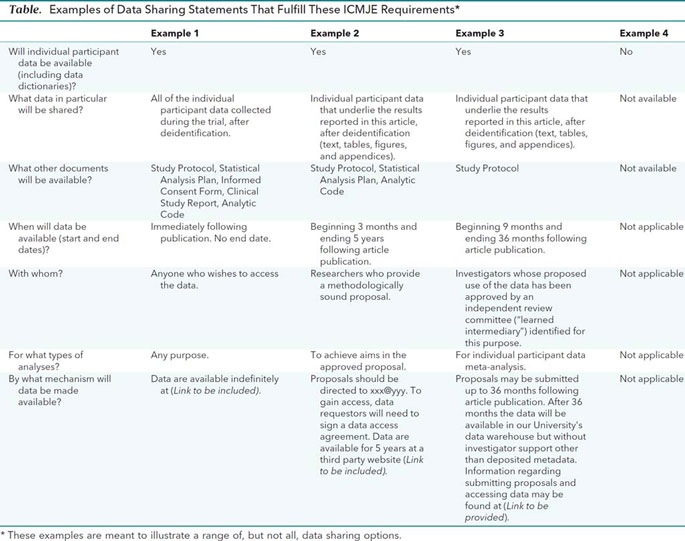
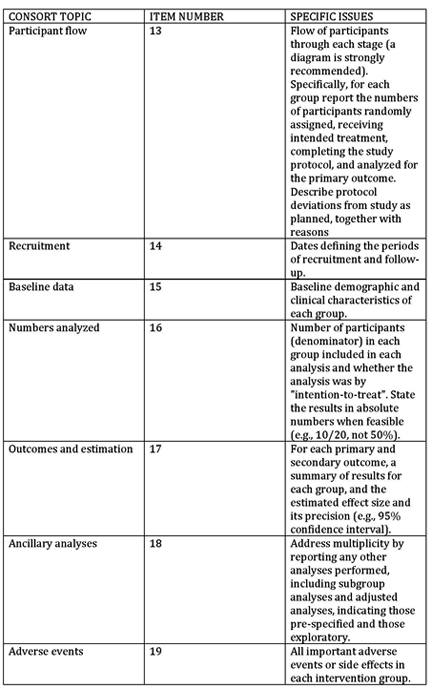
Trials is experimenting with a new way of structuring study protocols for randomised trials. The simple innovation is to include all 51 SPIRIT headings and item identifiers within the protocol itself. Readers will then benefit from the ability to search by item identifier, which are contained within curly brackets.
How do I submit a study Protocol reporting a clinical trial?
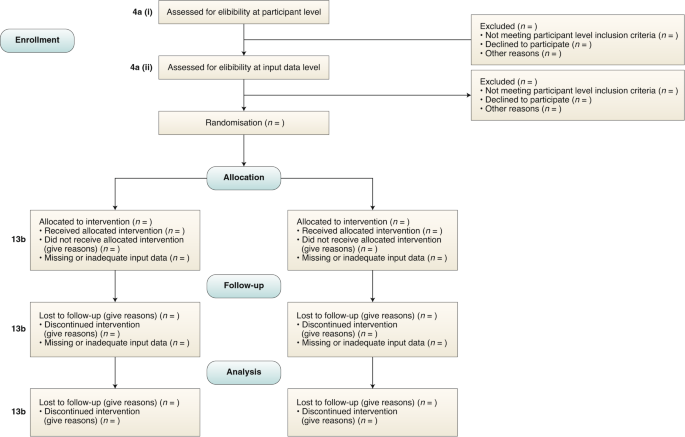
Study protocols reporting a clinical trial can be formatted for submission to Trials in two ways: By following the guidance set out in our structured study protocol template. This is the preferred option if you have not yet started writing your manuscript. By submitting a populated SPIRIT checklist and SPIRIT figure alongside your manuscript.
When will a study Protocol article be considered?
Study protocol articles will generally only be considered for proposed or ongoing trials that have not completed participant recruitment at the time of submission.
Can NIH applicants use a template to write clinical protocols?
NIH applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Phase 2 or 3 clinical trials that require Investigational New Drug applications (IND) or Investigational Device Exemption (IDE) applications
|
Guide to Performing Peer Review for Clinical Trial Protocol Articles
Clinical Trial Protocol Articles. All Future Science Group journals process article submissions via ScholarOne Manuscripts. For. |
|
Dose Escalation Study Design Fictional Manuscript
4 sept. 2019 The protocol specified 100 mg/m2 of Ender-G twice a day for 4 weeks administered intravenously for the first cohort. Successive cohorts were ... |
|
Study protocol: Antibiotics for Children with Severe Diarrhoea
ABCD trial protocol version 9.0 |
|
A Clinical Investigation Evaluating Efficacy of a Full- Thickness
Estimated duration for the main protocol (e.g. from start of The preparation and submittal for publication of manuscripts containing the study. |
|
E 3 Structure and Content of Clinical Study Reports Step 5
TABLE OF CONTENTS FOR THE INDIVIDUAL CLINICAL STUDY REPORT8 ... 9.7 Statistical Methods Planned in the Protocol and Determination of Sample Size.......16. |
| Factorial Study Design Fictional Manuscript |
|
HHS Public Access
Depression; obesity; randomized controlled trial; problem solving therapy; medication changes according to the study medication protocol (Appendix. |
|
SPIRIT 2013 Checklist: Recommended items to address in a clinical
prospective multi-center study protocol for diagnostics and follow up of peripheral nerve injuries. Trial registration. (page 13 of the manuscript). |
|
Study Protocol CTN-0093: Validation of a Community Pharmacy
25 mai 2021 Funded by: National Institute on Drug Abuse (NIDA) ... description the study protocol |
|
Dragon 1 Protocol Manuscript: Training Accreditation
Dragon 1 Protocol Manuscript: Training Accreditation |
|
Preparing a Study Protocol Article - Wellcome Open Research
All protocols for randomised clinical trials must be registered and follow the SPIRIT editing by Wellcome Open Research before publication and a manuscript |
|
Clinical Trial Protocol - ClinicalTrialsgov
Clinical Trial Protocol Clinical Trial No AEZS-108-050 Clinical Phase III Type of Study Therapeutic confirmatory Title Randomized controlled study comparing |
|
CLINICAL TRIAL PROTOCOL A Phase II - ClinicalTrialsgov
16 mai 2017 · with the requirements of this clinical study protocol and also in At the end of the trial, one or more manuscripts for joint publication may be |
|
Study Protocol - ClinicalTrialsgov
Protocol Title: A Phase 2, Multi-Center, Randomized, Placebo-Controlled Study of The preparation and submittal for publication of manuscripts containing the |
|
Merck Guidelines for publication of clinical trials
* In addition, for confirmatory (hypothesis-testing) trials, a manuscript will be submitted to a journal as soon as possible after the last patient's last visit occurs or last |
|
Clinical Trials Protocol Template - NIH Office of Science Policy
This Clinical Trial Protocol Template is a suggested format for Phase 2 or 3 clinical trials supported by the National Institutes of Health (NIH) that are being |
|
Registration of methodological studies, that is, ``research-on
our manuscripts, criticizing us for not having a priori regis- tered or published study protocol tered clinical trial protocols not publishing results years |
|
Trial protocol: a parallel group, individually randomized clinical trial
The funders had no role in study design, writing the study protocol, the decision to publish the study protocol, or preparation of the manuscript Availability of data |
|
Conduct of Clinical Trials Clinical Trial Results
trained on the clinical trial protocol, pharmaceutical prod- uct, and procedural tation; and (2) writes or revises the manuscript involving important intellectual |