clsi ep23 a guidelines
|
EP23-A : Laboratory Quality Control Based on Risk Management
Setting the standard for quality in medical laboratory testing around the world The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit membership organization that brings together the varied perspectives and expertise of the worldwide laboratory community for the advancement of a common cause: to foster excellence in laboratory |
|
CLSI EP23-A Laboratory Quality Control Based on Risk
Feb 14 2012 · EP23 – “The Right QC” • EP23 helps the lab director and the lab staff better understand their entire testing process from collecting the sample to reporting the result • EP23 stresses the importance of identifies and formalizes all of the other activities that labs do to ensure quality results |
Where can I find a ep23 workbook?
This document is available in electronic format only. You may also be interested in... Buy EP23 Workbook, A Practical Guide for Laboratory Quality Control Based on Risk Management, at CLSI. Find more standards documents in the CLSI Shop.
What is ep23-a?
EP23-A addresses the clinical laboratory path of workflow steps indicated by an “X.” For a description of the document listed in the grid, please refer to the Related CLSI Reference Materials section on the following page. SAM
What is the CLSI consensus process?
CLSI documents undergo periodic evaluation and modification to keep pace with advancements in technologies, procedures, methods, and protocols afecting the laboratory or health care. CLSI’s consensus process depends on experts who volunteer to serve as contributing authors and/or as participants in the reviewing and commenting process.
What does CLSI stand for?
Clinical and Laboratory Standards Institute (CLSI). Laboratory Quality Control Based on Risk Management; Approved Guideline. CLSI document EP23-A (ISBN 1-56238-767-7 [Print]; ISBN 1-56238-768-5 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2011.
Clinical and Laboratory Standards Institute
Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit membership organization that brings together the varied perspectives and expertise of the worldwide laboratory community for the advancement of a common cause: to foster excellence in laboratory
Consensus Process
Consensus—the substantial agreement by materially afected, competent, and interested parties—is core to the development of all CLSI documents. It does not always connote unanimous agreement, but does mean that the participants in the development of a consensus document have considered and resolved all relevant objections and accept the resulting ag
Commenting on Documents
CLSI documents undergo periodic evaluation and modification to keep pace with advancements in technologies, procedures, methods, and protocols afecting the laboratory or health care. CLSI’s consensus process depends on experts who volunteer to serve as contributing authors and/or as participants in the reviewing and commenting process. At the end o
Laboratory Quality Control Based on Risk Management; Approved Guideline
James H. Nichols, PhD, DABCC, FACB Ronald H. Laessig, PhD Wadid Sadek, PhD Sousan S. Altaie, PhD Ronalda Leneau, MS, MT(ASCP) Mitchell G. Scott, PhD Greg Cooper, CLS, MHA Jacob (Jack) B. Levine, MBA Ann E. Snyder, MT(ASCP) Paul Glavina W. Gregory Miller, PhD Liz Walsh, CLS, NCA Abdel-Baset Halim, PharmD, PhD, DABCC Robert Murray, JD, PhD Gitte Wenn
Introduction
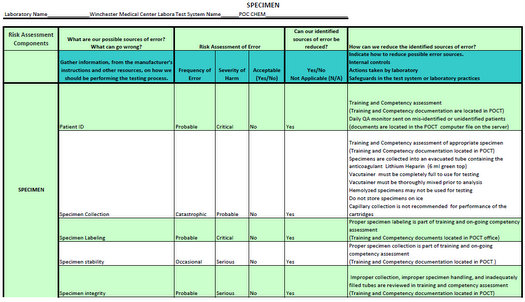
In this document, you will learn how to create a quality control plan (QCP) that is customized for your institution, facility, and laboratory, run your tests in an effective and efficient manner, so that you can improving patient care. clsi.org
You will learn:
How to compile information into How to determine if potential errors clsi.org
Laboratory Quality Control Based on Risk Management; Approved Guideline
note: This document may not satisfy the requirements of all regulatory, accreditation, or certification bodies. clsi.org
Laboratories need to comply with all applicable requirements
in the development of their QCPs. Scope This document describes good laboratory practice for developing and maintaining a QCP for medical laboratory testing using internationally recognized risk management principles. An individual QCP should be established, maintained, and modified as needed for each measuring system. The QCP is based on the perfo
|
EP23-A™: Laboratory Quality Control Based on Risk Management
Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit |
|
CLSI EP23-A Laboratory Quality Control Based on Risk
٢٢ ربيع الأول ١٤٣٣ هـ • CLSI document EP23 provides instruction on developing an appropriate QCP ... – Regulatory and accreditation requirements. – Measuring system ... |
|
EP23™
١٥ محرم ١٤٤٥ هـ Clinical and Laboratory Standards Institute (CLSI). Laboratory Quality Control Based on Risk Management. 2nd ed. CLSI guideline EP23 (ISBN ... |
|
Quality Planning with CLSI EP23-A
٢١ ربيع الأول ١٤٣٧ هـ Quality Control. Like the CLIA requirements COLA laboratories must establish a QC program for all tests performed in the lab. COLA |
|
EP23-A™ - Laboratory Quality Control Based on Risk Management
Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit |
|
Risk Management at the Point of Care: CMS IQCPs and the CLSI
CLSI Document EP23. • Laboratory Quality Control Based on Risk Management;. Approved Guideline (EP23-A™). • James H. Nichols PhD |
|
Presentation Title
١٨ صفر ١٤٣٩ هـ CLSI Guideline EP23 – A. Clinical and Laboratory Standards Institute ... Safety aspects — Guidelines for their inclusion in standards. ICH ... |
|
CLMA Body of Knowledge 2013
Related CLSI Documents. (as of June 2013). EP12-A2. EP23-A™ and EP23-A-WB. (EP23 workbook). GP11-A. GP27-A2. GP29-A2. GP31-A. The Key to Quality™. Laboratory |
|
Starting Soon CLSI Updates: Guidance Documents
https://www.whitehatcom.com/POCWebMtgs/Slides/Dr_Nichols_CLSI_Updates_090723.pdf |
|
CLSI TRAINING COURSES CATALOG
test kits and equipment following CLSI guidelines EP05 |
|
EP23-A™: Laboratory Quality Control Based on Risk Management
Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit |
|
CLSI EP23-A Laboratory Quality Control Based on Risk
14 Feb 2012 Management; Approved Guideline. Luann Ochs ... EP23 IS NOT about reducing quality control ... CLSI document EP23 provides instruction. |
|
Evolving Clinical Laboratory Management Through Implementation
12 May 2016 CLSI Guideline EP23 – A. Clinical and Laboratory Standards Institute. Laboratory quality control based on risk management. |
|
Quality Planning with CLSI EP23-A
1 Jan 2016 Explain the updated IQCP regulations put forth by CLIA ... waived testing requirements must be ... EP23-A Workbook. www.clsi.org. |
|
EP23™: Laboratory Quality Control Based on Risk Management 1st
Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit |
|
ALL COMMON CHECKLIST INDIVIDUALIZED QUALITY CONTROL
5 May 2015 Laboratories may develop their own model for designing an IQCP or use the Clinical and Laboratory. Standards Institute (CLSI) Guideline EP23-A ... |
|
DEPARTMENT OF HEALTH & HUMAN SERVICES
4 Nov 2011 EP-23 is the product of the CLSI and CLIA partnership: The CLSI standards document development utilizes a consensus process among affected ... |
|
DEPARTMENT OF HEALTH & HUMAN SERVICES
4 Nov 2011 Office of Clinical Standards and Quality/ Survey & Certification Group ... EP-23 is the product of the CLSI and CLIA partnership: The CLSI ... |
|
Risk Management at the Point of Care: CMS IQCPs and the CLSI
Recognize CLSI EP23 guideline as a resource for risk management. 3. Describe how to develop an Individualized Quality. Control Plan to meet the new CLIA |
|
Quality Control Based on Risk Management and Its Role in Quality
1 Oct 2013 CLSI document EP23 provides guidance on developing an appropriate IQCP that will: – Save 'me and money. – Use electronic and/or integrated QC ... |
|
EP23-A™: Laboratory Quality Control Based on Risk - CLSI
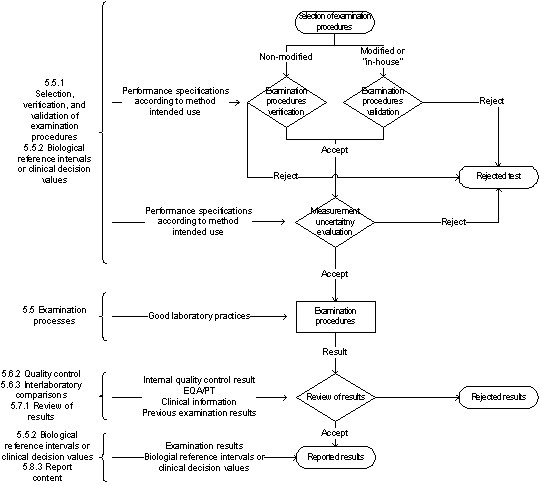
Clinical and Laboratory Standards Institute document EP23-A—Laboratory Quality Control Based on Risk Management; Approved Guideline provides guidance to laboratories on the development of quality control plans for measuring systems |
|
CLSI EP23-A Laboratory Quality Control Based on Risk - CDC
14 fév 2012 · Management; Approved Guideline Luann Ochs EP23 IS NOT about reducing quality control CLSI document EP23 provides instruction |
|
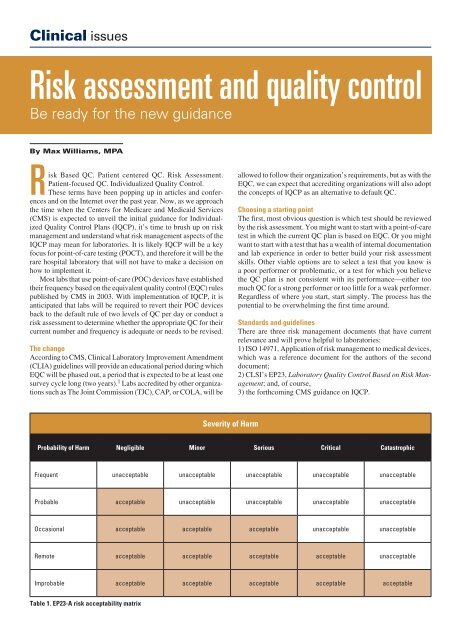
Risk management - IFCC
12 mai 2016 · CLSI Guideline EP23 – A Clinical and Laboratory Standards Institute Laboratory quality control based on risk management Approved guideline |
|
Quality Planning with CLSI EP23-A
1 jan 2016 · Quality Control Like the CLIA requirements, COLA laboratories must establish a QC program for all tests performed in the lab COLA, like CLIA, |
|
Risk Analysis QC Plans: Principles and Priorities - QCNet
CLSI EP23 CLSI C24 ISO 15198 ISO 22367 Manufacturers Laboratories SEVERITY - Provide instructions for “safe Key Guidance from ISO 14971 |
|
Ep23 A Laboratory Quality Control Based On Risk 339132 - Blue Bus
339132The Rong Regulation: Conflicts between NABL 112, CLSI EP23 Ep23 A Laboratory mean, SD derivation but global guidelines strongly recommend |
|
Risk management - Lab Quality Confab
1 oct 2013 · management system (QMS) framework • Risk management and CLSI document EP23TM • CMS requirements and the use of EP23 concepts |















![ISO CrossWalk Web - [PDF Document] ISO CrossWalk Web - [PDF Document]](https://europepmc.org/articles/PMC4071183/bin/alm-34-274-g001.jpg)