colligative properties of water in botany
|
Colligative properties of water
The colligative properties of solutions consist of freezing point depression boiling point elevation vapor pressure lowering and osmotic pressure n These properties ideally depend on changes in the entropy of the solution on dissolving the solute which is determined by the number of the solute molecules or ions but does not depend on their st |
|
Colligative Properties
If colligative properties depend on the amount of the solute in the solvent then the equations defining them must include a concentration term and sure enough they do Over the next few pages you will be introduced to the equations in the context of the specific properties but for now simply note the similarities in structure for the equat |
Why do ice and water vapor have colligative properties?
The rationale for these colligative properties is the increase in entropy on mixing solutes with the water. This extra entropy provides extra energy available in the solution, which has to be overcome when ice or water vapor (neither of which contains a non-volatile solute) b is formed.
What are colligative properties of electrolyte solutions?
The colligative properties of solutions consist of freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure. "Solutes simply dilute the solvent;..The colligative properties follow directly from this..." "It is the past treatment of electrolyte solutions that is not ideal, not the behavior of the solutions"
Is vapor pressure a colligative property?
The best way to demonstrate the importance of colligative properties is to examine the consequences of Raoult's law. Raoult found that the vapor pressure of the solvent escaping from a solution is proportional to the mole fraction of the solvent. But the vapor pressure of a solvent is not a colligative property.
Concentration and Colligative Properties
If colligative properties depend on the amount of the solute in the solvent, then the equations defining them must include a concentration term, and sure enough, they do. Over the next few pages you will be introduced to the equations in the context of the specific properties, but for now, simply note the similarities in structure for the equat
ΔP = χP0
0% pure solvent Since we know that vapor pressure is a surface phenomenon, we see that one obvious reason for the reduction in vapor pressure is that there are fewer surface locations to put a molecule. So the solvent will have to have a reduction in vapor pressure because fewer molecules are present to leave the surface. The plot of mole f
Colligative Property 2—freezing point depression is explained
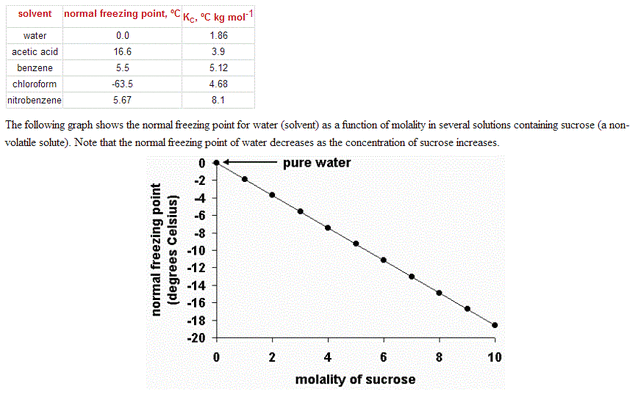
Freezing point depression is again thermodynamic effect. In order to make ice, which is a pure crystal, extra work must be done to separate the solute from the solvent—NaCl from water, for example. This means you need to reduce the freezing point to thermodynamically drive the reaction. This has the advantage of allowing for the deicing of st
|
Colligative properties of water
The colligative properties of solutions consist of freezing point depression, boiling point elevation, vapor pressure lowering and osmotic pressure Put another way, the solute stabilizes the water in the solution relative to pure water |
|
Water: Structure and Properties - University of Pennsylvania
Selected physical properties of water are given in Table 1 To put these in context , biology, occurring in protein folding, protein binding, enzyme catalysis, ion |
|
BSc-Part-II(BOTANY) - RMLAU
Botany B Sc II year Paper I: Diversity of Angiosperms: Systematics, Plant and water relationship, colligative properties of water, free energy concept Water |
|
BISC 367 Plant Physiology
water, one mole of sucrose is placed in a volumetric flask, dissolved in water and A significant colligative property of dissolved solutes to plant physiologists |
|
Water ascent in tall trees: does evolution of land plants rely - Esalq
16 oct 2003 · The literature and botanical textbooks published in the last four decades give sap composition, the features of the xylem wall and the hydraulic coupling of the Colligative properties of simple solutions Science 194(4265): |
|
L_7_General_Ed_Science_Stream - AICTE
Dilute solution, colligative properties, Raoult's law, relative lowering of vapour Analysis of an organic mixture containing two solid components using water, Elementary Knowledge of the following plants (Botanical names, families, parts |