difference between acid hydrolysis and base hydrolysis
What is hydrolysis in chemistry and physiology?
Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The other reactants, and the products of hydrolysis, may be neutral molecules, as in most hydrolyses involving organic compounds, or ionic molecules, as in hydrolyses of salts, acids, and bases.
Why is acidic hydrolysis common in the hydrolysis of esters?
Acidic hydrolysis is common in the hydrolysis of esters. Esters are compounds that form by the reaction of carboxylic acids with alcohol. When esters hydrolyze in the presence of an acid, it results in the formation of carboxylic acid and alcohol. Acidic hydrolysis also helps in the hydrolysis of nitriles and acetals.
What are the disadvantages of acidic hydrolysis?
One drawback of acidic hydrolysis is the controlling of reaction conditions in order to prevent side reactions and unwanted by-products. Another difficulty is handling highly corrosive and hazardous acids involved in the reaction. Basic hydrolysis is the use of a base to break down the chemical bond.

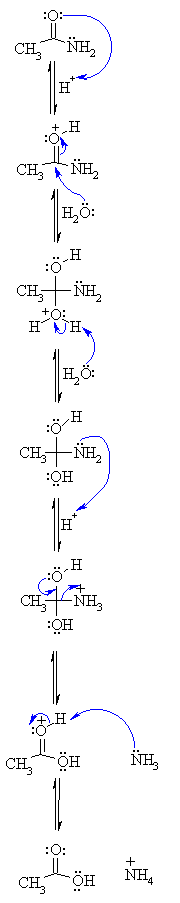
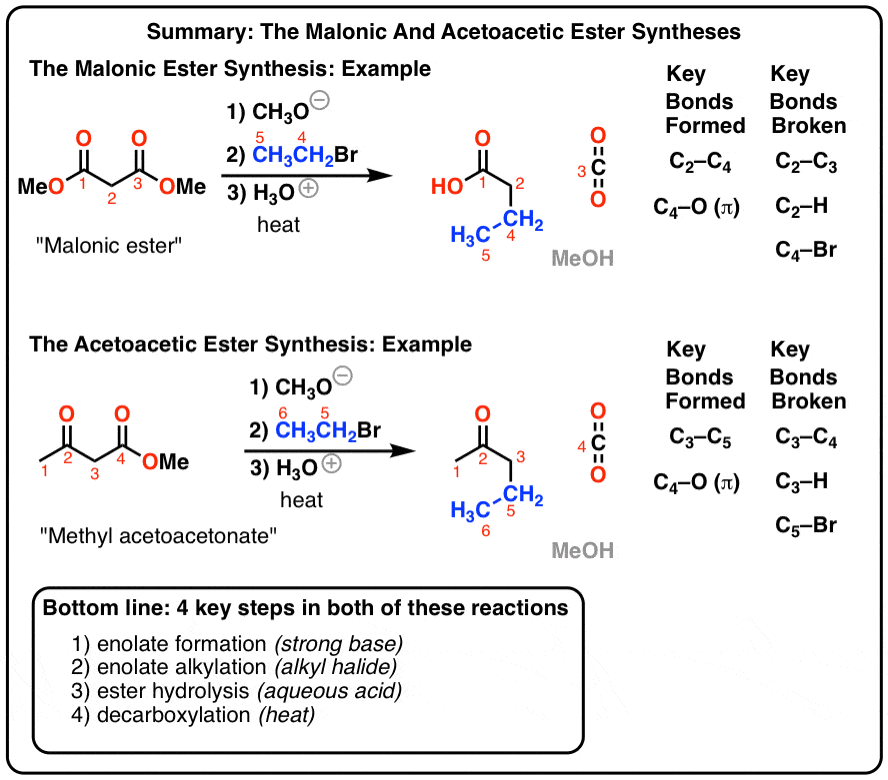
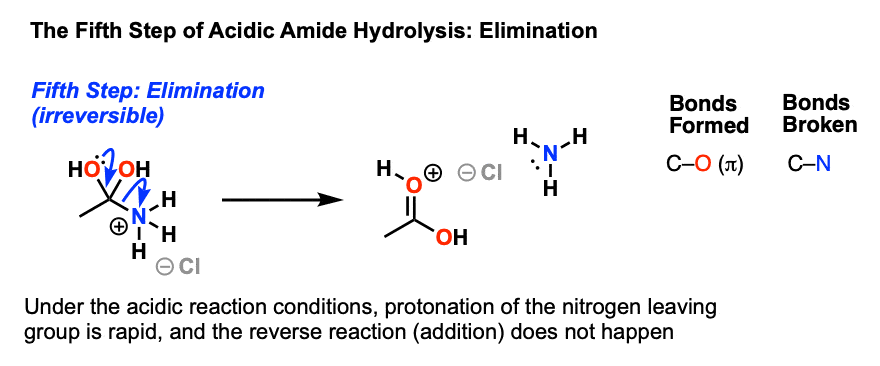
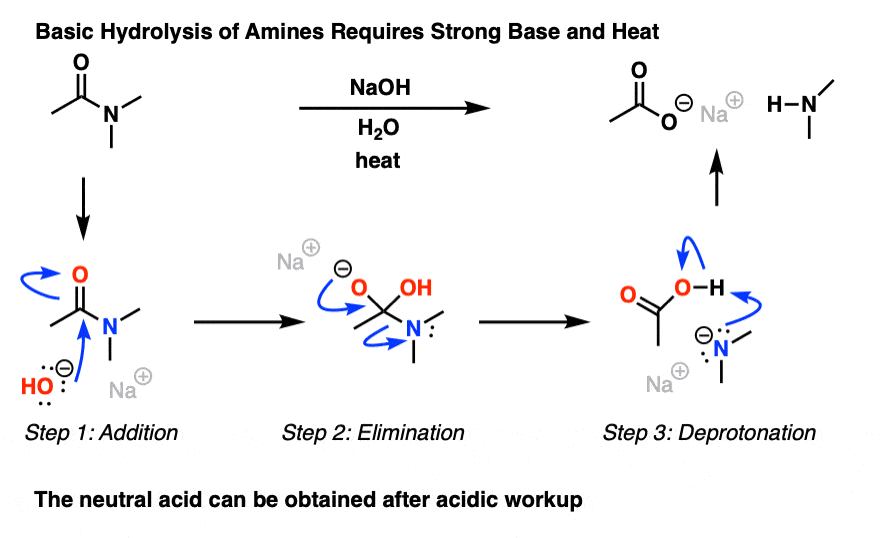
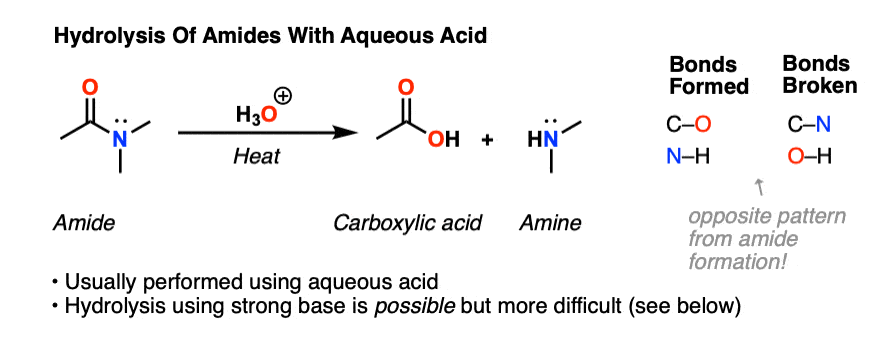
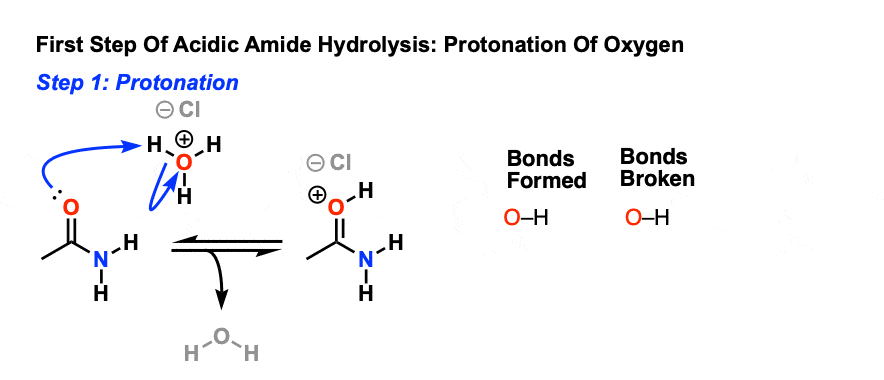
Acid and base-catalyzed hydrolysis of amides Organic chemistry Khan Academy

Hydrolysis and Dehydration Synthesis Reactions

Hydrolysis and Dehydration Synthesis
|
Acid and Alkaline Hydrolysis Extraction of Non-Extractable
The further purpose was to compare the difference between NEPPs obtained with alkaline and acid hydrolyses and to determine the optimal extraction method of |
|
HYDROLYSIS 2016.pdf
Hydrolysis reactions of organic substrates are ubiquitous (common) in the environment. mechanisms account for neutral acid and base hydrolysis. |
|
The Composition and Antioxidant Activity of Bound Phenolics in
7 dic 2020 by acid hydrolysis alkaline hydrolysis |
|
Mechanism of Substitution Reactions in Complex Ions. VII. Base
plexes and to compare the rates to those of acid hydrolysis (4). Experimental. Preparation of Compounds .—The compoundsused were. |
|
Biorefinery of the Olive Tree—Production of Sugars from Enzymatic
1 sept 2020 from Enzymatic Hydrolysis of Olive Stone Pretreated ... xylitol lactic acid and so forth |
|
Reaction Rate of the Alkaline Hydrolysis of Ethyl Acetate
The difference in the activation energies of the forward and reverse reaction rates was calculated from the experi- mental data. At lower temperatures this |
|
Kinetics and Mechanism of Base Hydrolysis of (Dimethyl Sulphoxide
for the base hydrolysis and the first-order rate constants of the acid base and that the charge difference between the complex and its conjugate. |
|
Ligand Displacement Reactions in Octahedral Complexes- Acid
Where ligand L is the leaving group present in the complex Base hydrolysis reactions may be defined as the reactions in which a hydroxo complex is ... |
|
Comparison and Optimization of Saccharification Conditions of
13 mar 2018 i.e. alkaline pre-treatment and acid hydrolysis |
|
Dilute-acid Hydrolysis of Cellulose to Glucose from Sugarcane
After cellulose-lignin was soaked in a solution of 1.5 % w/v NaOH |
|
Comparison of the Effects of Acid and Base Hydrolyses on Hydroxy
ence of cyclopropane acids For these reasons, the following study was done to compare the effects of acid and base hydrolysis on the fatty acid composition of |
|
HYDROLYSIS
It is generally observed that base catalyzed hydrolysis favours P-O or P-S cleavage, whereas neutral or acid catalyzed hydrolysis favours C-O or C-S cleavage |
|
Lecture 6: Hydrolysis Reactions of Esters and Amides
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions; • account for the So what is it that drives the reaction forward to product? |
|
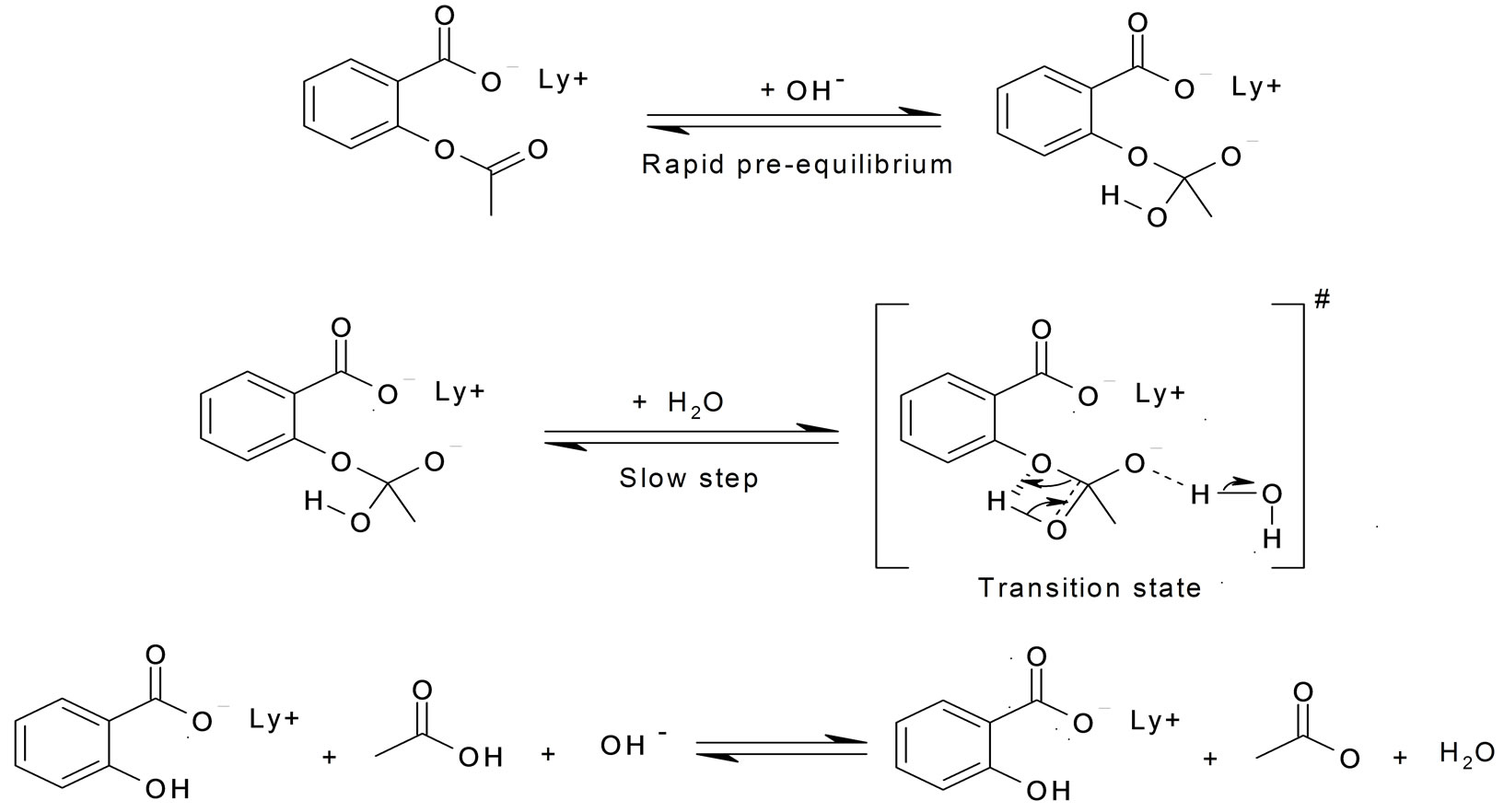
Mechanisms of Lactone Hydrolysis in Acidic Conditions
3 jui 2013 · A parallel work addresses the neutral and base-catalyzed hydrolysis of needs to be corrected for the difference between solvation enthalpies |
















![acid base catalysed Ester hydrolysis - [PDF Document] acid base catalysed Ester hydrolysis - [PDF Document]](https://ars.els-cdn.com/content/image/3-s2.0-B9780123877840000080-f08-081-9780123877840.jpg)