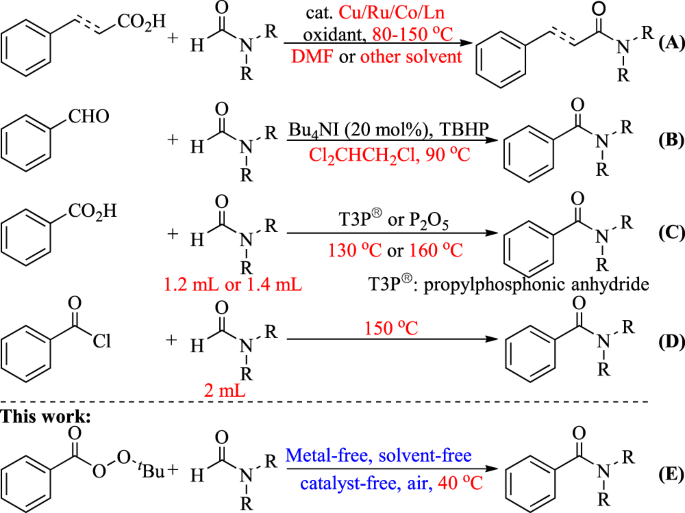
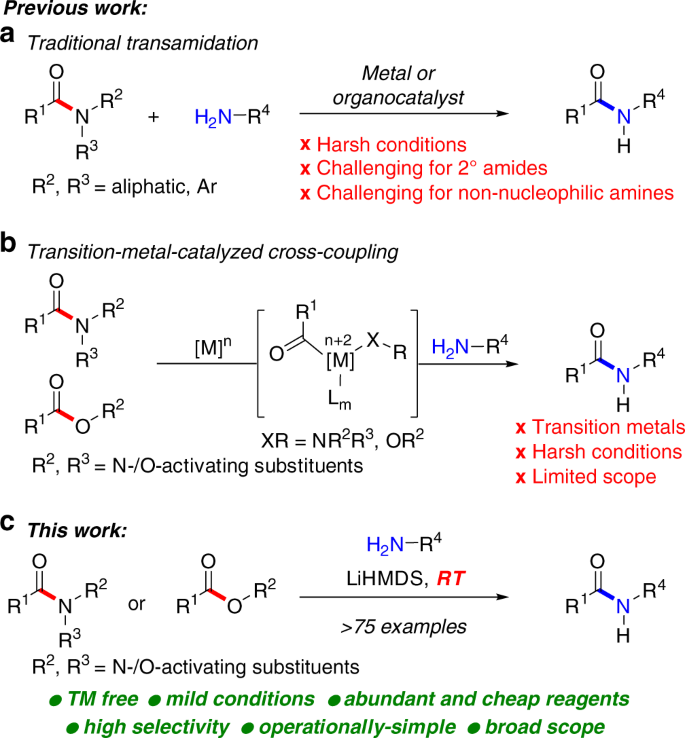
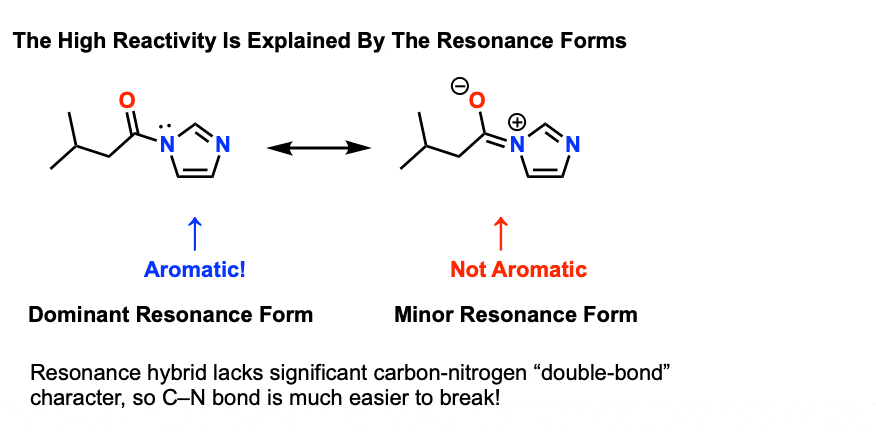
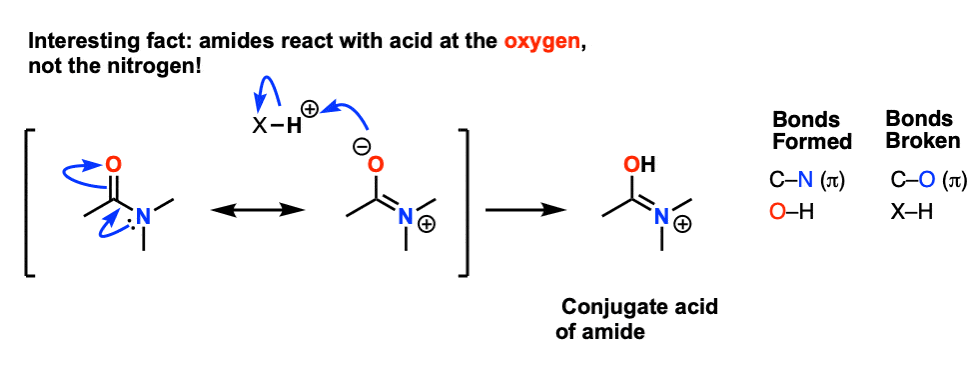
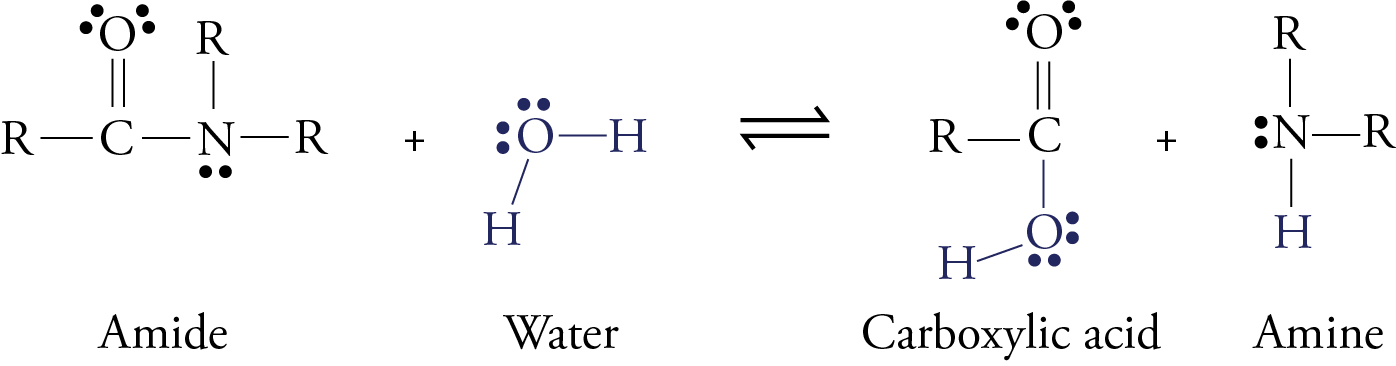amide hydrolysis products
|
HEATS OF HYDROLYSIS OF AMIDE AND PEPTIDE BONDS
The classi- cal procedure involves the determination of the heats of combustion of the reactants and the products the heat of reaction being equal to the sum |
|
PDF
The absorbance of the amide in 0 1 M hydrochloric acid was measured at 230 nm where the absorbances of the hydrolysis products are small The rate |
|
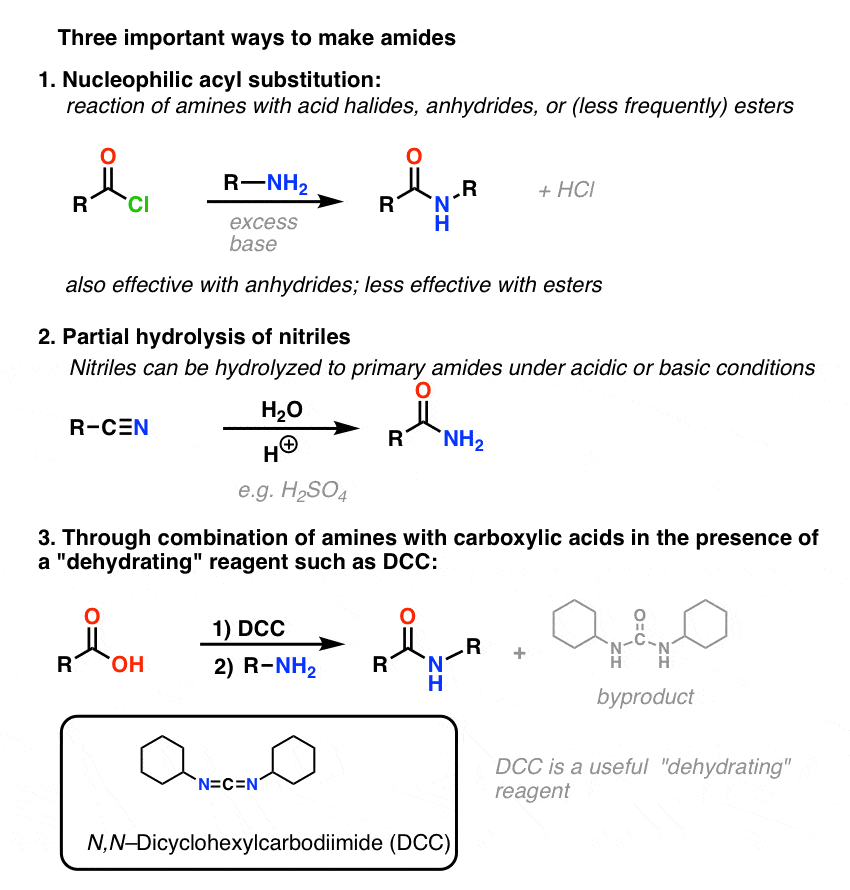
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the products of amide synthesis and hydrolysis reactions • Learn some of |
|
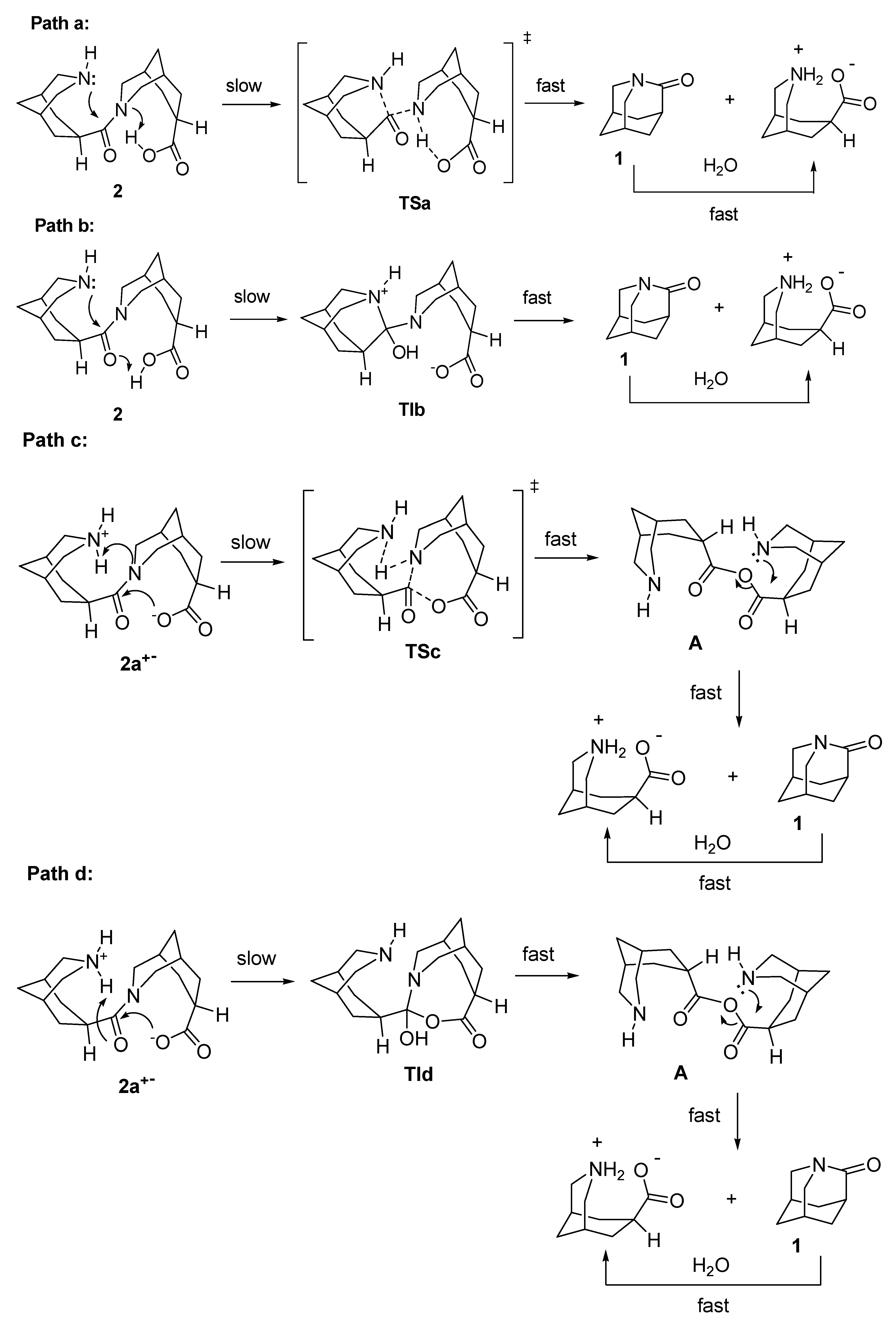
On the hydrolysis mechanisms of amides and peptides
15 mai 2018 · “tetrahedral” complex) and the products P (“acid + amine”) varies Theoretical study of base-catalyzed amide hydrolysis: gas- and aqueous-phase |
|
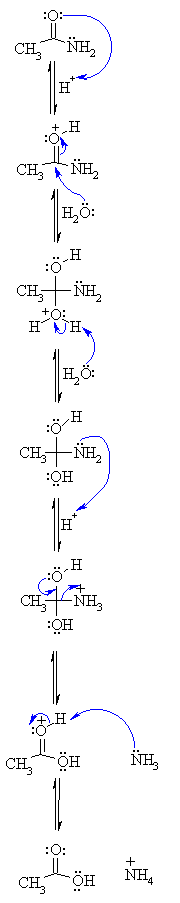
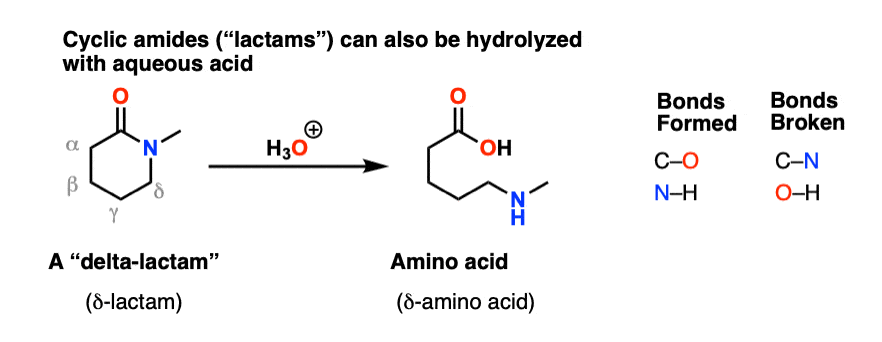
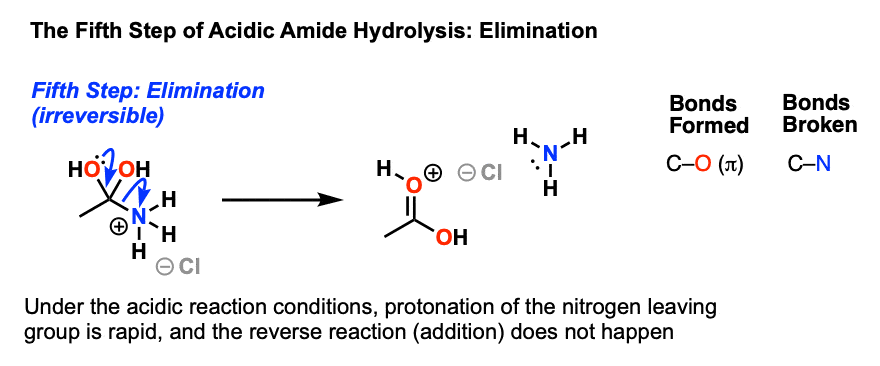
76 Hydrolysis of Amides The reversibility of this reaction means that
When diet sodas are consumed strong acid of the stomach can hydrolyze the ester linkage and peptidases in the small intestine can hydrolyze the amide bond Draw |
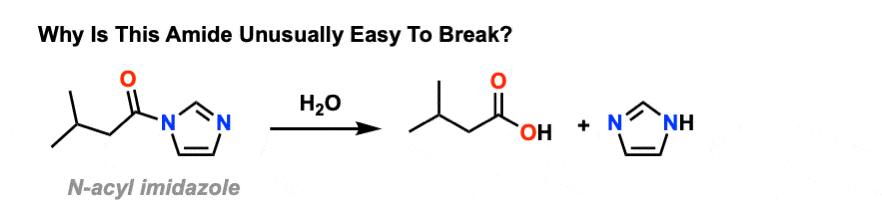
What are the products of amide degradation?
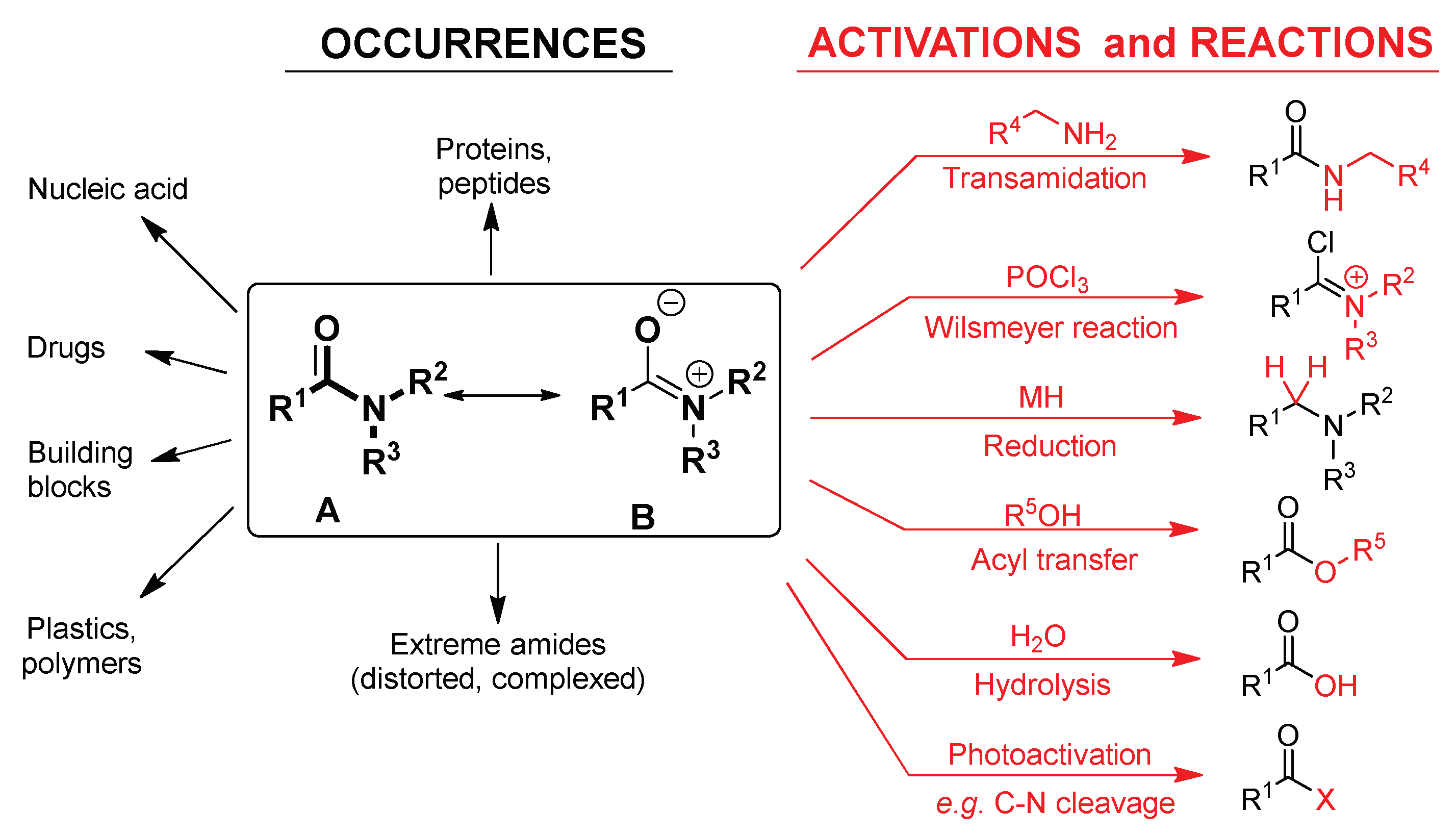
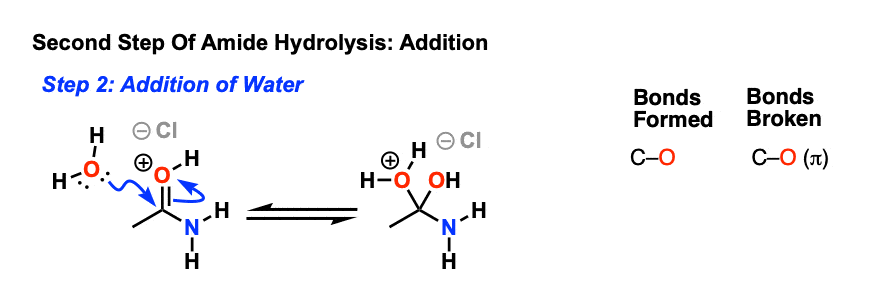
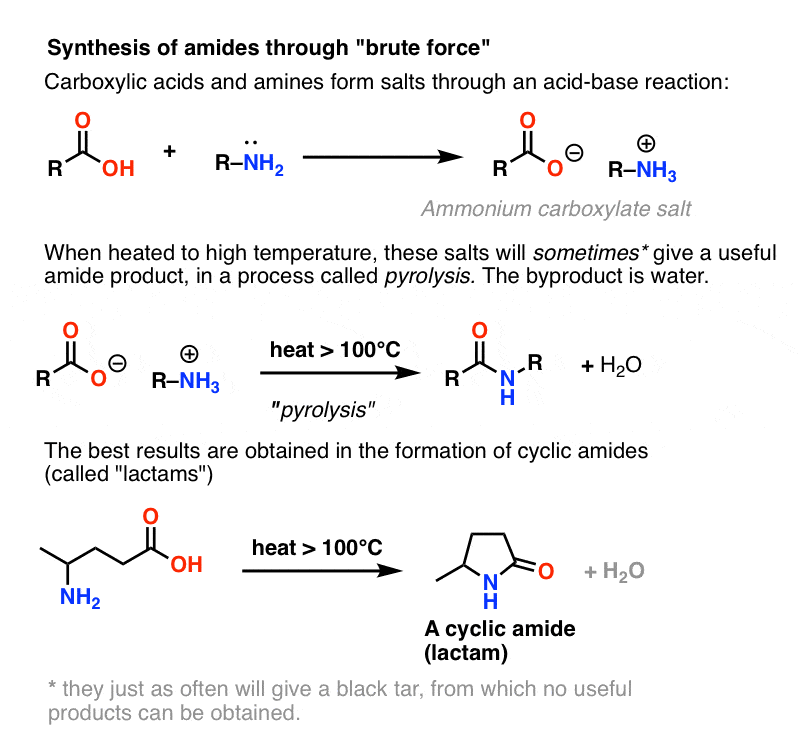
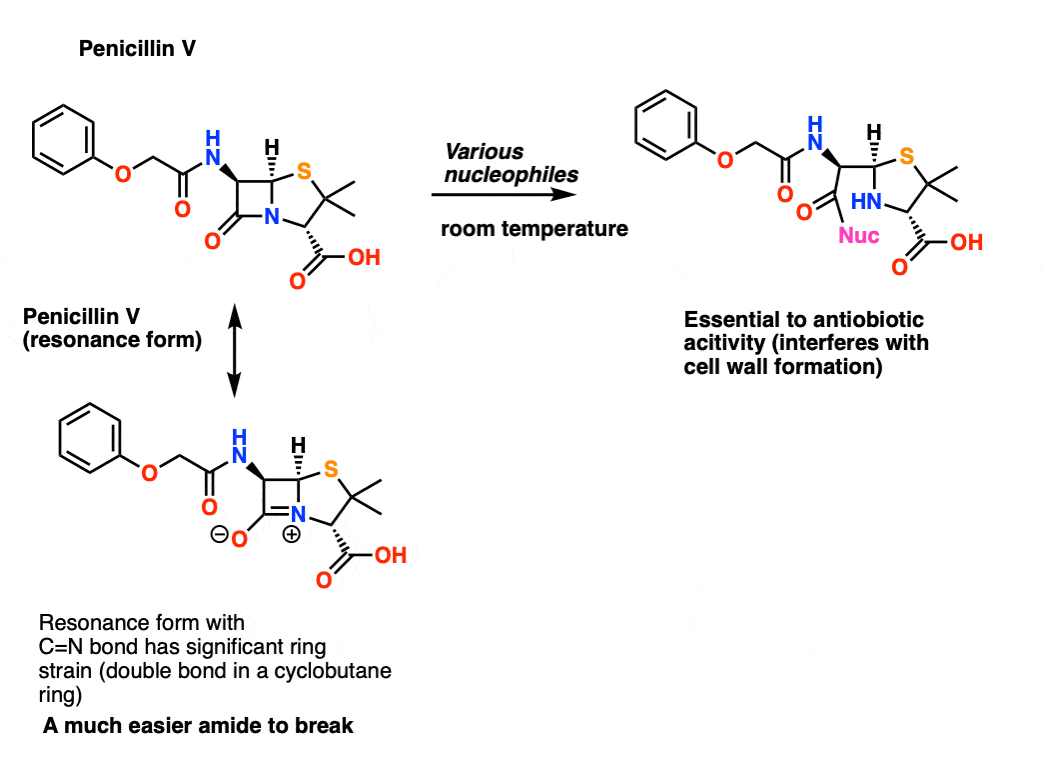
Amides can be hydrolyzed into a carboxylic acid and ammonia or an amine by heating in an acidic or basic aqueous solution.
In both cases, acid-base reactions occurring with the products make the overall reaction irreversible.What are the products of amide hydrolysis?
The hydrolysis of an amide produces a carboxylic acid and ammonia or an amine.13 août 2022
What are the products of amides?
Amides hydrolyse in hot alkali as well as in strong acidic conditions.
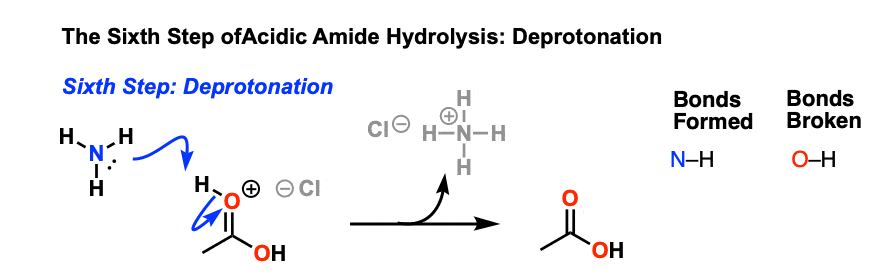
Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia.Amides are hydrolysed when heated with aqueous acid or alkali and give carboxylic acid and ammonia.
Amides are hydrolysed when heated with aqueous acid or alkali and give carboxylic acid and ammonia.
AMIDES:Amides can be prepared by following methods;Carboxylic acids react with aq. By the reaction of acid halides or acid anhydrides with ammonia.Reactions of amides are given below;1.Hydrolysis.
|
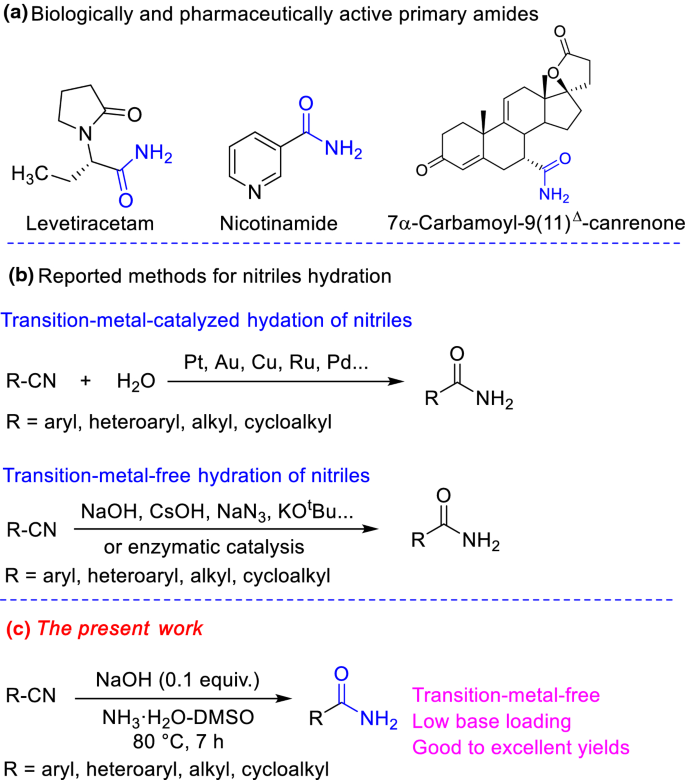
Base-promoted hydrolysis of amides at ambient temperatures
The respective products retained their specific radioactivity Table I Yields Obtained in the Hydrolysis of Amides with Potassium ten-Butoxide-Water". |
|
Theoretical Study of Base-Catalyzed Amide Hydrolysis: Gas-and
isomerization and the subsequent breakdown to products. The reaction is very exothermic in the gas Even though acids can promote amide hydrolysis |
|
Carboxylic acid participation in amide hydrolysis. Competition
of the hydrolysis products prevents the intermediate formationof an imide. The rapid hydrolysis of amides through a reaction involving. |
|
Models of zinc-containing proteases. Catalysis of cobalt(III
enhanced the rate of metal-promoted amide hydrolysis by more than 30 times over that due to the metal alone. The initial product of amide hydrolysis was |
|
Cobalt (111) -Promoted Hydrolysis of Chelated Glycine Amides
Abstract: A variety of N-0 chelated glycine amide and peptide complexes of the type [CoN4(glyNR1R2)]3+ Products of Co(trien)-Promoted Amide Hydrolysis. |
|
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the products of amide synthesis and hydrolysis reactions. |
|
Sans titre
7.6 Hydrolysis of Amides Write the amide hydrolysis reaction for the following amides: ... More attention has been directed to the methanol product. |
|
Catalytic Efficiencies in Amide Hydrolysis. The Two-Step
reactants rather than to products |
|
Kinetics and mechanism of amide acetal hydrolysis. Carbon-oxygen
or oxygen loss exists. Results. Products. The question of concern is which bond does cleave initially in amide acetal hydrolysis. Carbon-nitrogen fission. |
|
Catalysis of Amide Hydrolysis and Formation under Neutral
ester and amide substrates involvesformation of a cysteine 5-acyl (to form hydrolysis products or products from capture by amine). |
|
On the hydrolysis mechanisms of amides and peptides
15 mai 2018 · amide, formamide, hydrolysis rate law, mechanism, peptide, square root reaction “tetrahedral” complex) and the products P (“acid + amine”) |
|
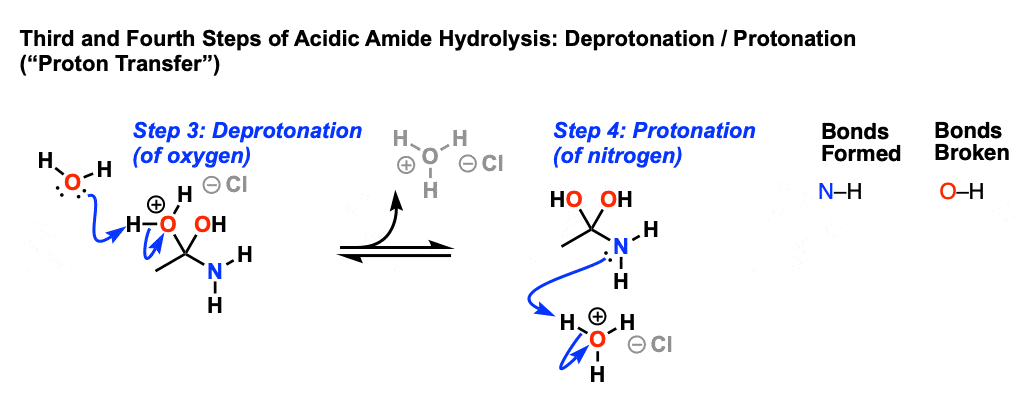
76 Hydrolysis of Amides
The reversibility of this reaction means that an amide can hydrolyze to form an amine and a carboxylic acid |