comparing the reaction rates of primary
How do you differentiate between average and instantaneous reaction rates?
Differentiate between average and instantaneous reaction rates using a plot of concentration versus time A rate is a measure of how some property varies with time. Speed is a familiar rate that expresses the distance traveled by an object in a given amount of time.
What is the difference between a wage and a chemical reaction?
Wage is a rate that represents the amount of money earned by a person working for a given amount of time. Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much product is produced, by the reaction in a given amount of time.
What is the rate of a chemical reaction?
Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much product is produced, by the reaction in a given amount of time. The rate of reaction is the change in the amount of a reactant or product per unit time.
What is the rate of a reaction stoichiometry?
The rate of a reaction may be expressed as the change in concentration of any reactant or product. For any given reaction, these rate expressions are all related simply to one another according to the reaction stoichiometry. The rate of the general reaction
AP Chemistry Curriculum Framework
This lesson supports the following learning objectives: 1. Big Idea 4: Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanations of phenomena. 1.1. 4.1 The student is able to design and/or interpret the results of an experiment regarding the factors (i.e., temperatur
Objectives
By the end of this demonstration, students should be able to 1. Describe how temperature affects the rate of a chemical reaction. 2. Describe how concentration of reactants affects the rate of a chemical reaction. 3. Describe how particle size or surface area affects the rate of a chemical reaction. teachchemistry.org
Chemistry Topics
This demonstration supports students’ understanding of 1. Kinetics 2. Chemical Reactions 3. Reaction Rates 4. Indicators of Chemical Change teachchemistry.org
Materials
250 ml beakers, 7Alka-Seltzer tablets, 8Water, 900 mlOptional: food coloring teachchemistry.org
Safety
Students should wear proper safety gear during chemistry demonstrations. Safety goggles and lab apron are required. teachchemistry.org
Teacher Notes
Alka-Seltzer is composed of three solid ingredients – acetylsalicylic acid (aspirin), citric acid, and sodium bicarbonate.When added to water, the citric acid reacts with the sodium bicarbonate, which is a base. Carbon dioxide is produced, which causes the bubbles.Students should know that in order for a reaction to occur, reactant particles must collide with enough energy to overcome the activation energy of that specific reaction.The following factors affect the rate of a reaction: teachchemistry.org
Demonstration Procedure
Introduction:The purpose of the demonstration is to show the effect of temperature, concentration, and surface area on the rate of a chemical reaction. Have students brainstorm about ways you could

GCSE Chemistry

Rates of Reaction

Rate law and reaction order Kinetics AP Chemistry Khan Academy
|
Reactivity of isocyanates with urethanes: Conditions for allophanate
22 juil. 2019 isocyanate-alcohol reaction was also confirmed the rate of reaction ... primary amine followed by NMR analysis of the degradation products ... |
|
Kinetics of the reaction of carbon dioxide ( CO2) with cyclic amines
1-MPZ1-. EPZ |
|
Quantitative comparison of photo-and electron-beam
Comparing UV and EB polymerizations initiated with equivalent primary radical of the UV and EB reactions be the same but the average rate of radical ... |
|
E 9 Statistical Principles for Clinical Trials Step 5
The role of statistics in clinical trial design and analysis is trials is discussed but is not a primary focus of this guidance. |
|
Heterologous primary and booster COVID-19 vaccination
13 déc. 2021 the immune response as compared to homologous vaccination ... The safety primary outcome was 7-day reactogenicity: reactions were ... |
|
Quantitative Comparison of Photo- and Electron-beam
Comparing UV and EB polymerizations initiated with equivalent primary radical of the UV and EB reactions be the same but the average rate of radical ... |
|
A Comparative Kinetics Study of CO2 Absorption into Aqueous
primary amines as well as the alkyl chain length of tertiary amines have a significant Ltd. (U.K.) to study the chemical reaction rates between CO2. |
|
RELATIVE REACTIONS RATES OF CHLORINE ATOMS WITH
for reaction at a primary carbon atom the values of the activation energies rates (d) and (Y) |
|
Chemrevise
Haloalkanes can be classified as primary secondary or tertiary depending Comparing the rate of hydrolysis reactions. Water is a poor nucleophile but it ... |
|
Janssen COVID-19 Vaccine EUA Fact Sheet for Healthcare Providers
5 mai 2022 The primary vaccination regimen for the Janssen COVID-19 Vaccine is a ... conducted under widely varying conditions adverse reaction rates. |
|
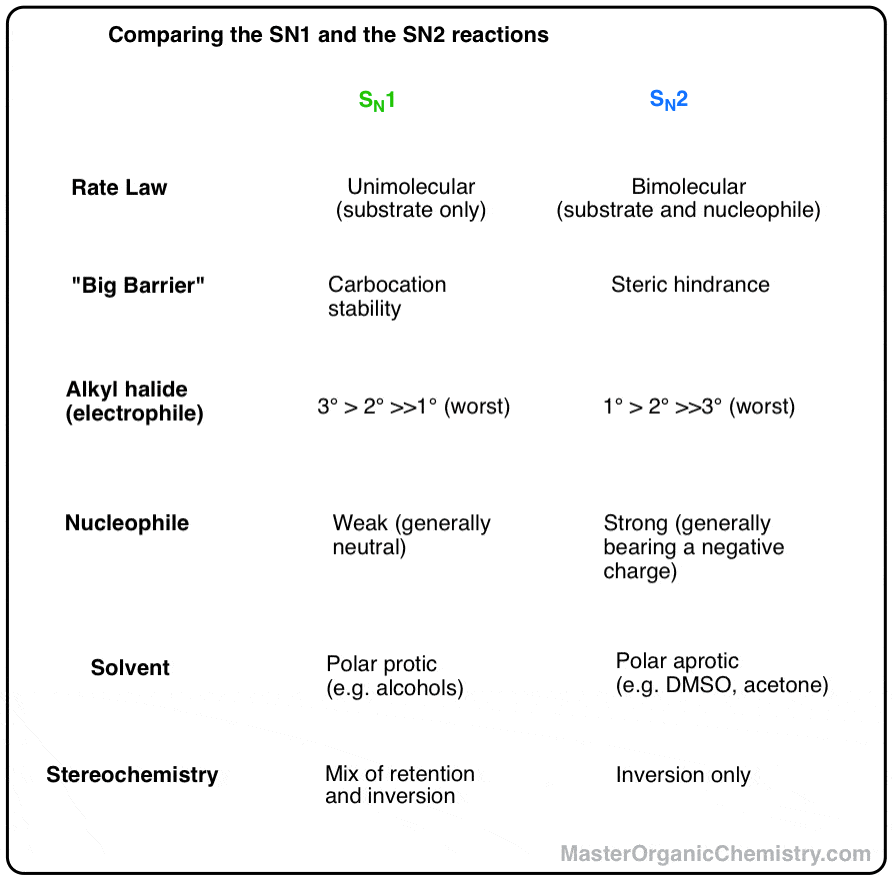
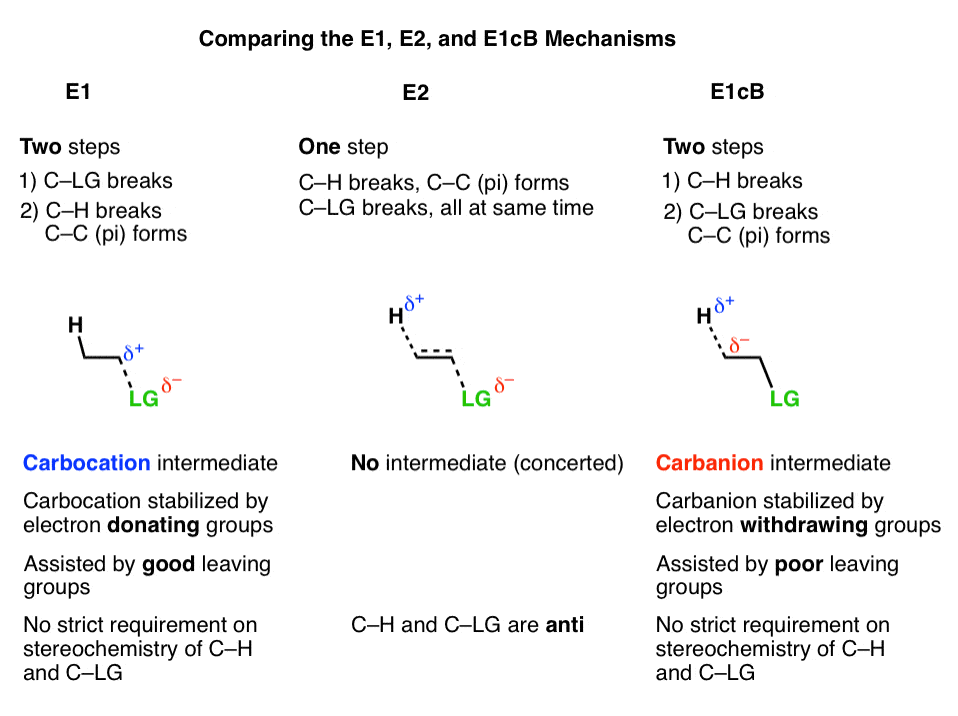
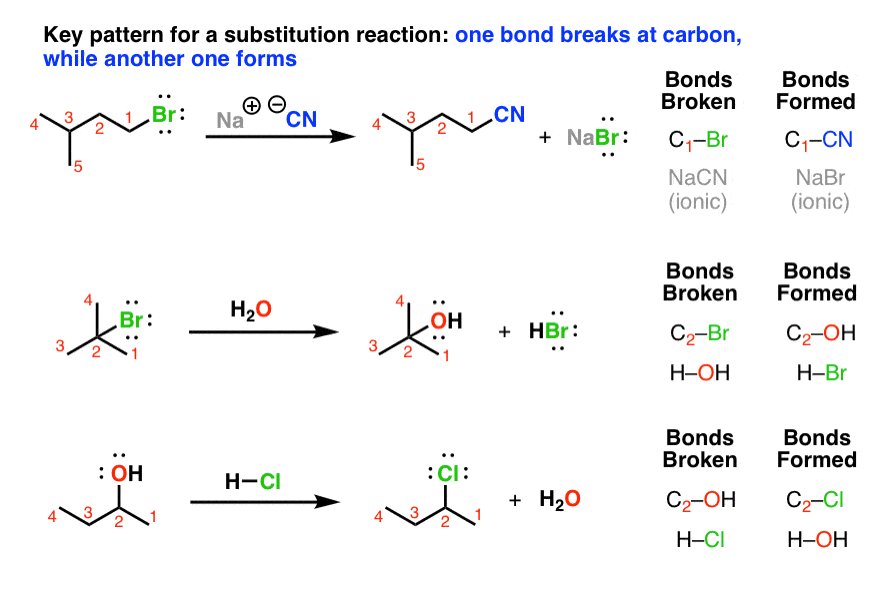
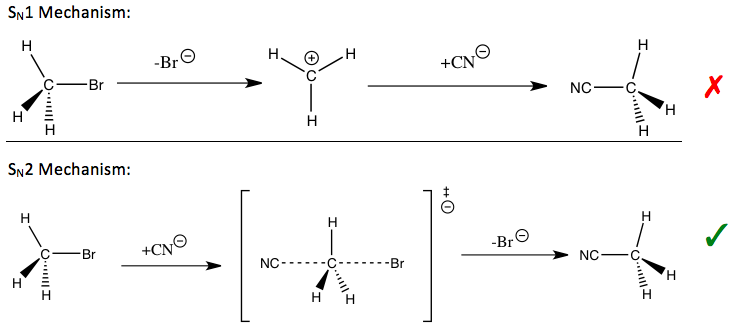
1 Chapter 11: Nucleophilic Substitution and Elimination Walden
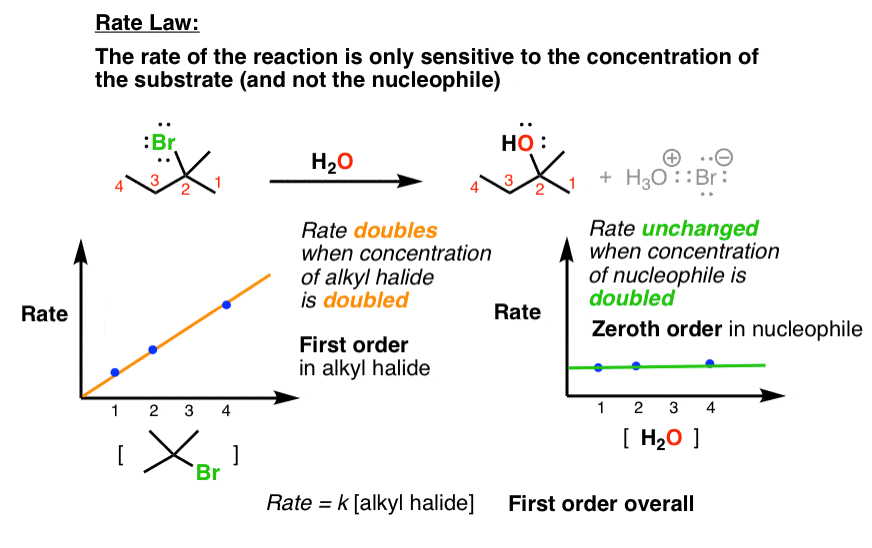
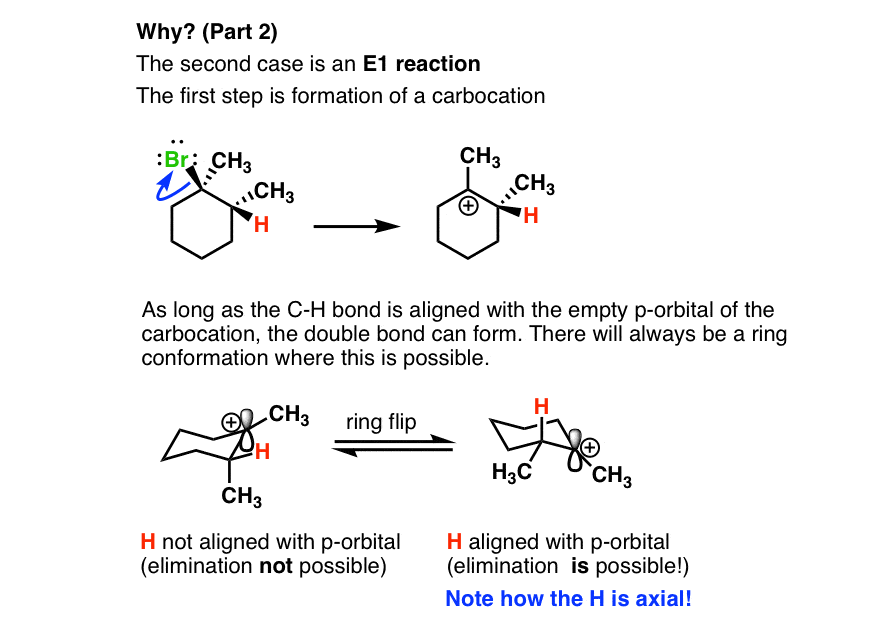
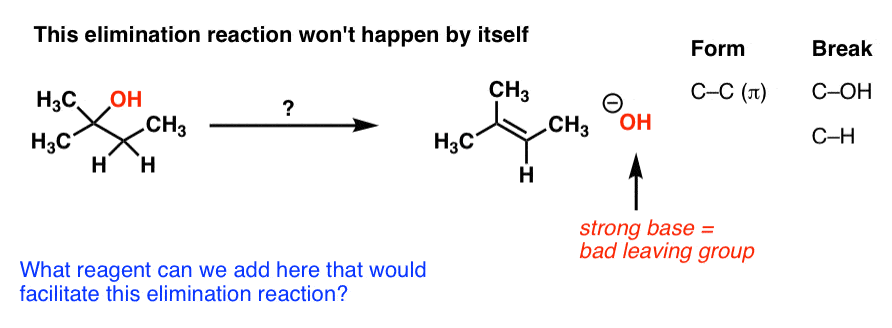
If [CH3-Br] is doubled, then the reaction rate may be doubled Nucleophilicity roughly parallels basicity when comparing nucleophiles Primary (1°) C R H R |
|
Chapter 17: Reaction Rates
describe and calculate the rates at which chemical reactions occur Expressing 2NaOH(aq) Comparing the two equations, it's evident that the reactions are similar These three elementary steps comprise the complex reaction 2O3 0 3O2 |
|
Kinetics of the reaction of carbon dioxide ( CO2) with cyclic - CORE
1-MPZ,1- EPZ,HEPZ and 3-MOPA have reaction rates similar to primary and secondary amines [in the region of 0-1000 s-1) Figure 4 Comparison of pseudo- first |
|
Kinetics of the reaction of carbon dioxide ( CO2) with cyclic amines
1-MPZ,1- EPZ,HEPZ and 3-MOPA have reaction rates similar to primary and secondary amines [in the region of 0-1000 s-1) Figure 4 Comparison of pseudo- first |
|
Elementary reactions - IOPscience
One of the aims of any book on chemical reaction rates must therefore reaction, known as elementary reactions, and will develop a simple model that allows Comparing equation 1 16 with equation 1 12, we see that simple collision theory |
|
Physical Chemistry 3: — Chemical Kinetics — - Uni Kiel
26 jui 2019 · 2 4 4 Comparison of first-order and second-order reactions Bunker 1966 D L Bunker, Theory of Elementary Gas Reaction Rates, Pergamon, |
|
Chapter 5
observed by comparing reactions rates in gas phase and in solution, and by Two main factors affect the rate of elementary reactions in solution: 1) The fact that |
|
Reaction Kinetics: The Iodine Clock Reaction - Bellevue College
2- (thiosulfate) with the I2 produced in the primary reaction: A comparison of the reaction rate with and without a catalyst will demonstrate catalytic action |