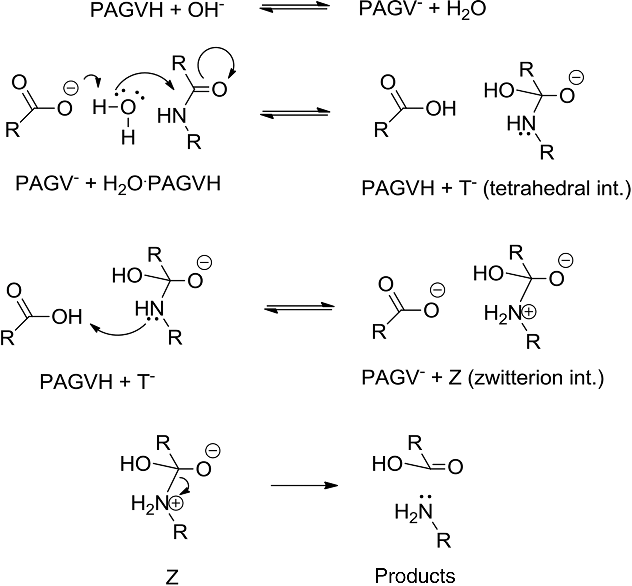
amide hydrolysis with hcl
|
Hydrolysis of Amides
HCl (1 equiv) was added The mixture was stirred for 2 h The HCl salt of R-amide (R)-3 was collected by filtration; yield: 30–47 ; >99 ee The R-amide (R)- |
Why is HCl used in hydrolysis?
The stages in the hydrolysis include preparation of materials, the process of hydrolysis and analysis of test results using Fehling and titrate with standard glucose solution.
HCl is used as a catalyst because it is cheaper than the enzyme that has the same function.What is the hydrolysis of amides with acid?
What is acid catalysed amide hydrolysis? It is a reaction of the amide with water in an acidic medium.
In an acidic medium, amide interacts with the water molecule to give a carboxylic acid and the salt of ammonia or amine salt.What does HCl do to an amide?
Hydrolysis under acidic conditions
Taking ethanamide as a typical amide.
If ethanamide is heated with a dilute acid (such as dilute hydrochloric acid), ethanoic acid is formed together with ammonium ions.
So, if you were using hydrochloric acid, the final solution would contain ammonium chloride and ethanoic acid.22 jan. 2023Taxonomy of Prokaryotes
The reaction for acetamide hydrolysis is CH3CONH2 + H2O → CH3COOH + NH3.
The NH3 renders the medium alkaline, causing the pH indicator phenol red to change a yellow-orange to a red or magenta colour. (Method of Greenberg et al., 1985).
|
THE KINETICS OF HYDROLYSIS OF THE AMIDE GROUP IN
Kinetic data are presented for the hydrolysis of L-asparagine to L-aspartic acid and ammonia in 0.2 to 1.0 N hydrochloric acid and L-asparaginylglycine to |
|
THE HA ACIDITY FUNCTION AND THE MECHANISM OF AMIDE
behavior of amides to aqueous hydrochloric acids because in addition to for a wider range of amide hydrolysis reactions and provide more general ... |
|
The Hydrolysis of Vitamin B12. Studies with Model Amides
amide are described and therates of hydrolysis of these and other model amides in aqueous hydrochloric acid-dioxane at 50° are recorded. Aquocobalamin has. |
|
Fatty Acid Amide Hydrolase Substrate Specificity
Endogenous oleamide and anandamide are hydrolyzed and inactivated by fatty acid amide hydrolase. (FAAH)17 |
|
Carboxylic acid participation in amide hydrolysis. Evidence that
Carboxylic Acid Participation in Amide Hydrolysis. Evidence That Separation of a Nonbonded Complex Can Be. Rate Determining. Ronald Kluger* and Jik Chin. |
|
Mild hydrolysis or alcoholysis of amides. Ti(IV) catalyzed conversion
aqueous hydrochloric acid converts this class of compounds into the corresponding carboxylic acids or esters. Thus heating the carboxamide in a solution of the |
|
Hydrolysis of Amides to Carboxylic Acids Catalyzed by Nb2O5
25 Dec 2020 KEYWORDS: Nb2O5 Catalyst Amide hydrolysis |
|
Dilute acid-catalyzed amide hydrolysis: efficiency of the N
protonated amide to show that hydrolysis via the latter species is not sufficient to support the overall observed acid-hydrolysis rate constant for amides. |
|
Untitled
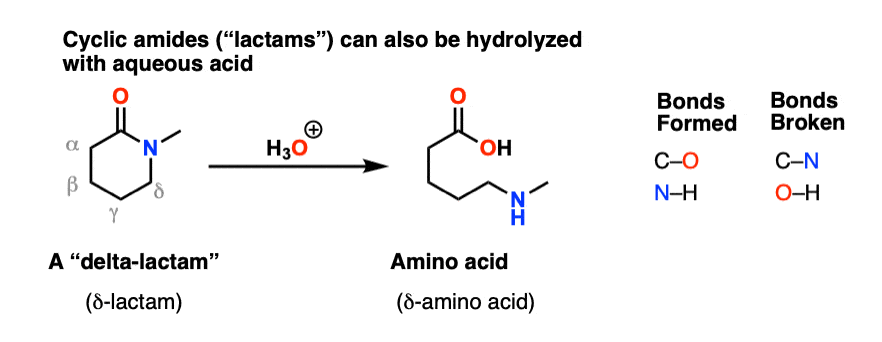
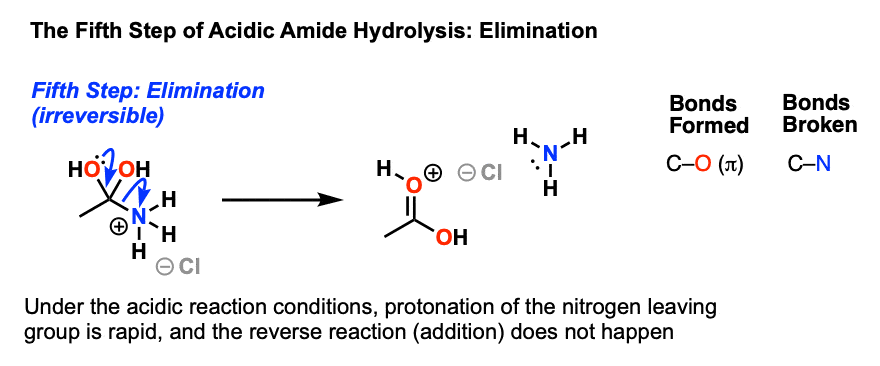
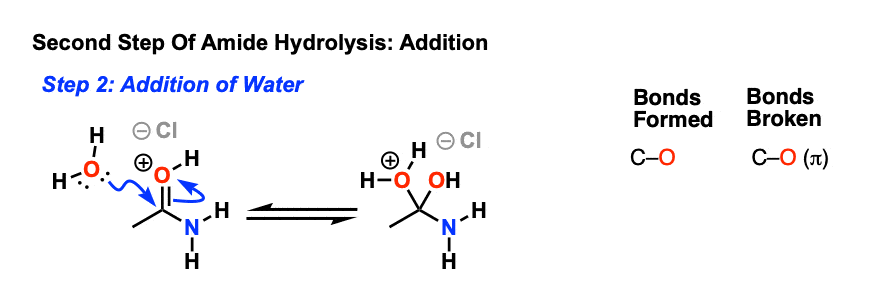
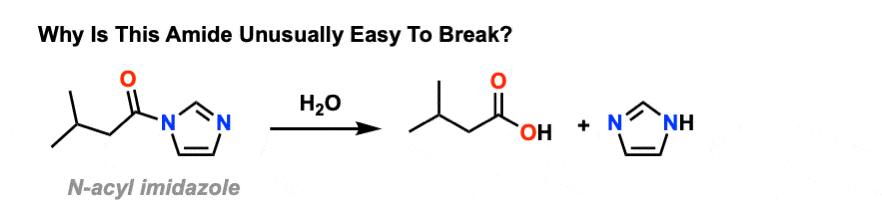
7.6 Hydrolysis of Amides. The reversibility of this reaction means that an amide can hydrolyze to form an amine and a carboxylic acid. |
|
THE HYDROLYSIS OF AMIDES IN THE ANIMAL BODY
rate of decomposition of urea by acid falls as the acidity is in- creased; the velocity of amide hydrolysis by acid on the other. |
|
Studies relating to the hydrolysis of aliphatic amides - CORE
falls conveniently into three divisions, dealing with ¥amino but~amide, malonic acid amides and oxalic acid ami des respectively A study of the hydrolysis of Y |
|
THE HYDROLYSIS OF AMIDES IN THE ANIMAL - ScienceDirect
rate of decomposition of urea by acid falls as the acidity is in- creased; the velocity of amide hydrolysis by acid, on the other hand, is roughly proportional to the |
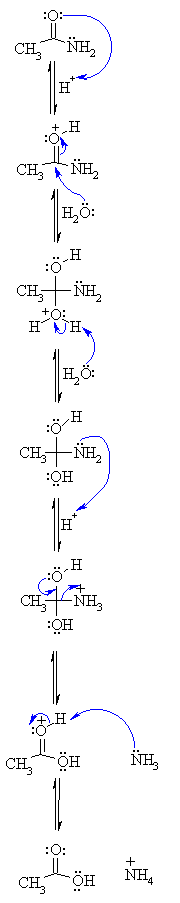










![A New Mechanism for Amide Hydrolysis - [PDF Document] A New Mechanism for Amide Hydrolysis - [PDF Document]](https://www.mdpi.com/molecules/molecules-23-02859/article_deploy/html/images/molecules-23-02859-g005.png)