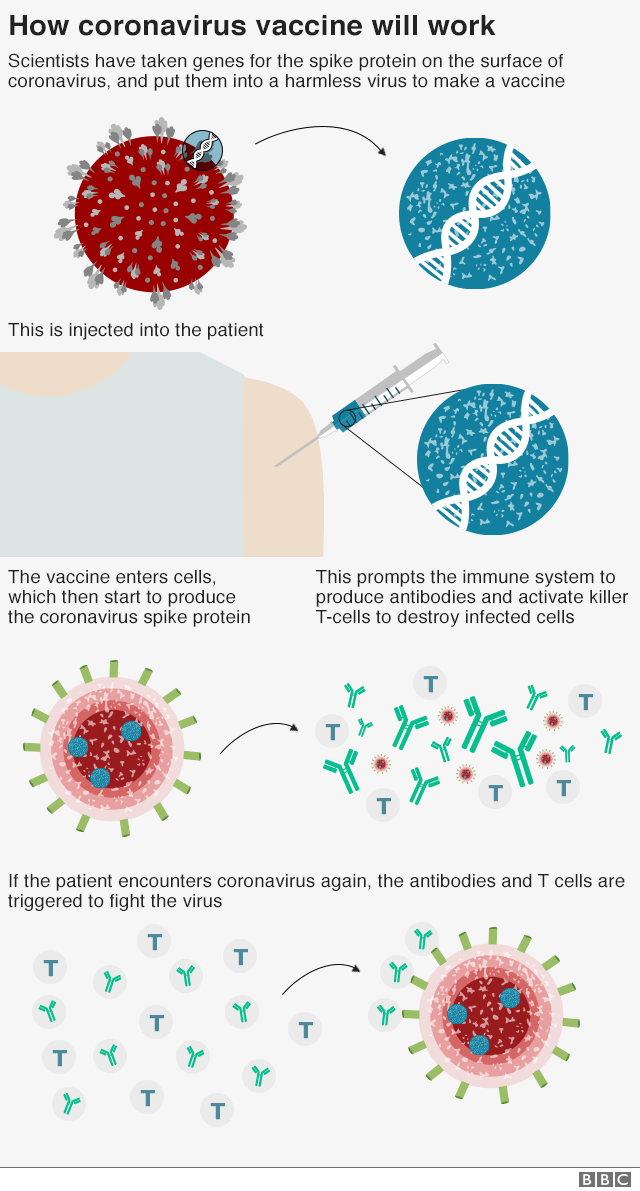
coronavirus vaccine update cdc
Is the COVID vaccine still effective?
It requires two shots, given 3 to 8 weeks apart.
Research done before the spread of the delta and omicron variants has shown that the vaccine is 90% effective at preventing mild, moderate and severe disease with COVID-19 .
For people age 65 and older, the vaccine is 79% effective.What are the possible side effects of the COVID-19 vaccine? You could experience soreness at the injection site, fatigue, headache, body aches, and fever.
Has the COVID vaccine been updated?
And unlike the spring booster that targeted people ages 60 and older, these updated vaccines are for everyone ages 6 months and older.
The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) approved the updated vaccines by Pfizer-BioNTech and Moderna in mid-September.
|
COVID-19 Vaccine safety updates
cdc.gov/coronavirus. COVID-19 Vaccine safety updates. Advisory Committee on Immunization Practices. (ACIP). June 23 2021. Tom Shimabukuro |
|
Update on Emerging SARS-CoV-2 Variants and COVID-19 vaccines
cdc.gov/coronavirus. Update on Emerging SARS-CoV-2. Variants and COVID-19 vaccines. Heather Scobie PhD |
|
ACIP Update: Thrombosis with thrombocytopenia syndrome (TTs
12 mai 2021 Update: Thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination ... CDC COVID-19 Vaccine Task Force. |
|
ACIP-COVID-19 Safety Update-January 27 2021
27 janv. 2021 Disease Control and Prevention (CDC) or the U.S. Food and Drug ... Update on anaphylaxis following COVID-19 vaccination. |
|
ACIP COVID-19 Vaccine Safety Update-March 1 2021
1 mars 2021 text message check-ins from CDC (daily 1st week; weekly thru 6 weeks; then 3 6 |
|
COVID-19 vaccine safety updates
1 sept. 2022 cdc.gov/coronavirus. COVID-19 vaccine safety update: Primary series in young children and booster doses in older children and adults. |
|
Pfizer-BioNTech COVID-19 Vaccine: Storage and Handling Summary
26 sept. 2022 General Information. Refer to CDC's Vaccine Storage and Handling Toolkit for best practices for vaccine storage and handling. |
|
COVID-19 vaccine safety updates: Primary series in children and
5 janv. 2022 COVID-19 vaccine safety updates: ... CDC COVID-19 Vaccine Task Force ... 12–15 years after Pfizer-BioNTech COVID-19 vaccination* (as of Dec ... |
|
Pfizer-BioNTech COVID-19 Vaccine Updated label Information • 6
20 juin 2022 CS321570-AP. 6 Months Through 4 Years of Age. Pfizer-BioNTech COVID-19 Vaccine. Updated Label Information. Vial label states Age 2y to <5y. |
|
Updates on COVID-19 and Pregnancy CDC Coronavirus Disease
Lead Maternal Immunization. ACIP Meeting. September 22 |
|
COVID-19 vaccine Safety update—January 2021 - CDC
27 jan 2021 · Vaccine Safety Datalink (VSD) surveillance update ▫ Update on anaphylaxis following COVID-19 vaccination ▫ Reports of deaths and |
|
Allocation of initial supplies of COVID-19 vaccine - CDC
Page 1 Redirect This content is located at the address below Please take a moment to update your bookmark: |
|
COVID-19 Vaccination Program Interim Playbook - CDC
29 oct 2020 · Agreement funding recipients (i e , “awardees”) should use this document to develop and update their COVID- 19 vaccination plans |
|
COVID-19 Vaccine Safety Update pdf icon[40 pages] - CDC
1 mar 2021 · V-safe update ▫ Vaccine Adverse Event Reporting System (VAERS) update ▫ Vaccine Safety Datalink (VSD) update ▫ Clinical Immunization |
|
Vaccinate with Confidence Tips for the Healthcare Team - CDC
CDC COVID-19 Response Healthcare personnel: A priority for COVID-19 vaccination safety data as they come in and provides regular safety updates |
|
CDC Covid-19 Vaccine
You may find similar content at the address below https://www cdc gov/vaccines/ acip/meetings/downloads/slides-2020-12/slides-12-19/05- · COVID-Clark-508 pdf |
|
Clinical Considerations for use of COVID-19 vaccines pdf - CDC
1 mar 2021 · Sign up to receive email updates when clinical considerations are updated: COVID-19 vaccines are administered intramuscularly as either a |
|
COVID-19 Vaccine Update - NCgov
il y a 6 jours · The Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP) decides who should be |
|
COVID-19 Vaccination Plan - TNgov
8 mar 2021 · 03/08/2021 Updates to Phase 1c based on CDC guidance Various other clarifications Addition of Janssen vaccine information MFiscus |
|
COVID-19 Vaccination Plan - Wisconsin Department of Health
Section 9: COVID-19 Vaccine Administration Documentation and Reporting and templates for mass clinics, and updated CDC guidance regarding the safe |