types of buffer solution with example pdf
|
Buffers Types of Buffer Solutions Solutions of Single Substance
The solution of any salt of a weak acid and a weak base e g ammonium acetate also shows buffering property Page 4 TYPES OF BUFFERS • There are two types |
What are the 3 types of buffer systems?
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
An example of a buffer solution is bicarbonate in blood, which maintains the body's internal pH.What is an example of an acidic and basic buffer?
The body's chemical buffer system consists of three individual buffers: the carbonate/carbonic acid buffer, the phosphate buffer and the buffering of plasma proteins.
While the third buffer is the most plentiful, the first is usually considered the most important since it is coupled to the respiratory system.What is a buffer with example?
Acidic buffer example: Mixture of acetic acid and sodium acetate.
Basic buffer example: Mixture of ammonium hydroxide and ammonium chloride.
|
Section 19.1. Acid-Base Buffer Solutions
species participating in the equilibrium. Example: addition of sodium acetate (CH3COONa or NaAc) to acetic acid (CH3COOH or HAc) solution. |
|
BUFFER SOLUTIONS
A common example would be a mixture of acetic acid and sodium acetate in solution. You can change the pH of the buffer solution by changing the ratio of acid to |
|
Buffer solutions and preparation of acidic and basic buffers.
Preparation of basic buffer: It is prepared by mixing equimolar solutions of weak base and its salt with strong acid e.g. NH4OH ammonium hydroxide. |
|
Buffers
instead as an equilibrium mixture of undissociated and dissociated species. For example in aqueous solution |
|
Buffering agents and Buffers
Bis-Tris is a popular gel sample and running buffer for various types of electrophoresis. Its use has also been reported in chromatography |
|
A Guide to Polyacrylamide Gel Electrophoresis and Detection
The protein sample may be prepared from type used should suit the properties of the protein ... buffers in the gels and electrode solutions (Wheeler. |
|
CHT™ Ceramic Hydroxyapatite Instruction Manual
Two types of CHT ceramic hydroxyapatite Type I and Type II |
|
A Guide to HPLC and LC-MS Buffer Selection
When samples contain ionizable compounds the mobile phase pH can be one of referred to as “Type-A” silica) |
|
?711? DISSOLUTION
monograph? for dosage forms administered orally. for example inlay tablets. ... buffered solution |
|
BUFFER SOLUTIONS
3- Calculate the pH of all sorts of buffer solutions 4- Know how to prepare all types of buffer solutions A common example would be a mixture of acetic |
|
Chapter 17 lecture notes - Cal State LA
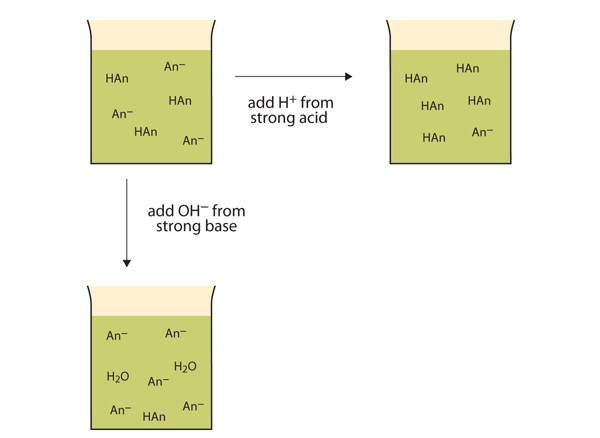
A buffer helps a solution maintain its pH when acid or base is pH of buffered solution adding 0 10 M HCl to 100 mL soln pair in which the acid and base forms of the concentration of ions in solution Examples AgCl(s) Ag+(aq) + Cl-( aq) |
|
Section 191 Acid-Base Buffer Solutions - George Christou
species participating in the equilibrium Example: addition of sodium acetate ( CH3COONa or NaAc) to acetic acid (CH3COOH or HAc) solution CH3COOH (aq ) |
|
Handout on Buffer Solutions - Carnegie Mellon University
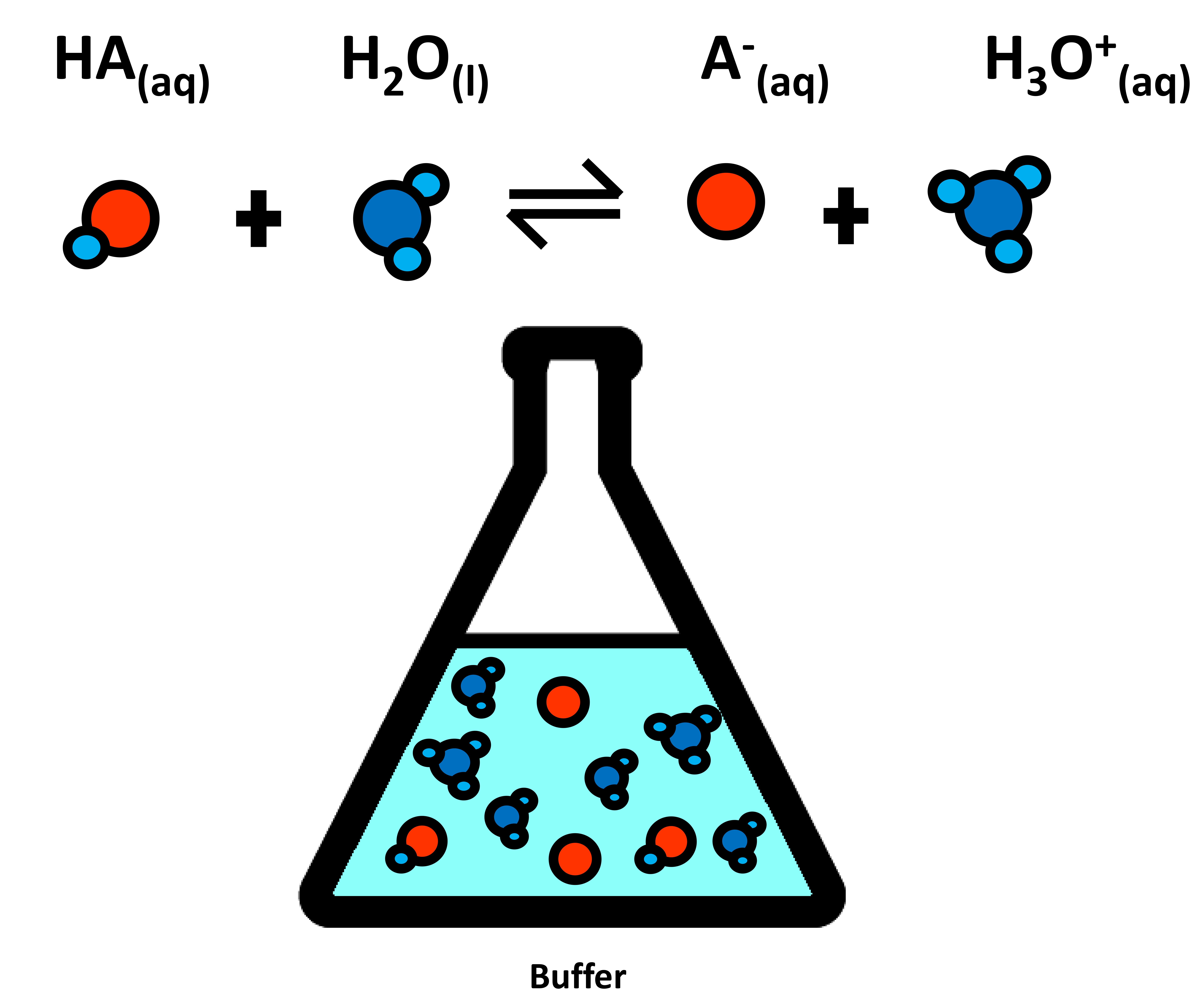
6 mar 2001 · To understand buffer solutions, you need to understand a number of concepts and then tie these The interesting species in the solution are then HA, A-, H3O+, and OH- We will use acetic acid (vinegar) as our example, |
|
Bufferpdf - Yengage
There are two types of buffer solutions These are: 0) Solution of Single Substance: The solution of the salt of weak acid and a weak base, e g , ammonium |
|
The Preparation of Buffers and Other Solutions: A - Sun Laboratory
amine-type buffer (e g , Tris) for an acid-type buffer (e g , phos- phate) Generally, this To a first approximation, the pH of a buffer solution is inde- pendent of the absolute Manual, Amersham Pharmacia Biotech, 1994) Incompatible salts |
|
The SOLUTION for All of Your Buffer Needs - The Wolfson Centre for
around the pKa for the system This rela- tionship is important in choosing a buffer For example, if a procedure calls for a pH of 3 75, formic acid (pKa = 3 75 at |
|
PH buffers Buffer capacity and buffering range
Introduction Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its Considering the following reaction (in this example During the classes, students will perform pH-meter calibration, titration of weak organic |
|
A buffer is a solution that resists changes in pH upon the addition of
Technical definition (How do you make one?): A buffer is composed of a mixture· of a weak acid its conjugate base (Sometimes a solution that is technically a buffer does NOT resist changes in pH What is meant by "buffering capacity"? |