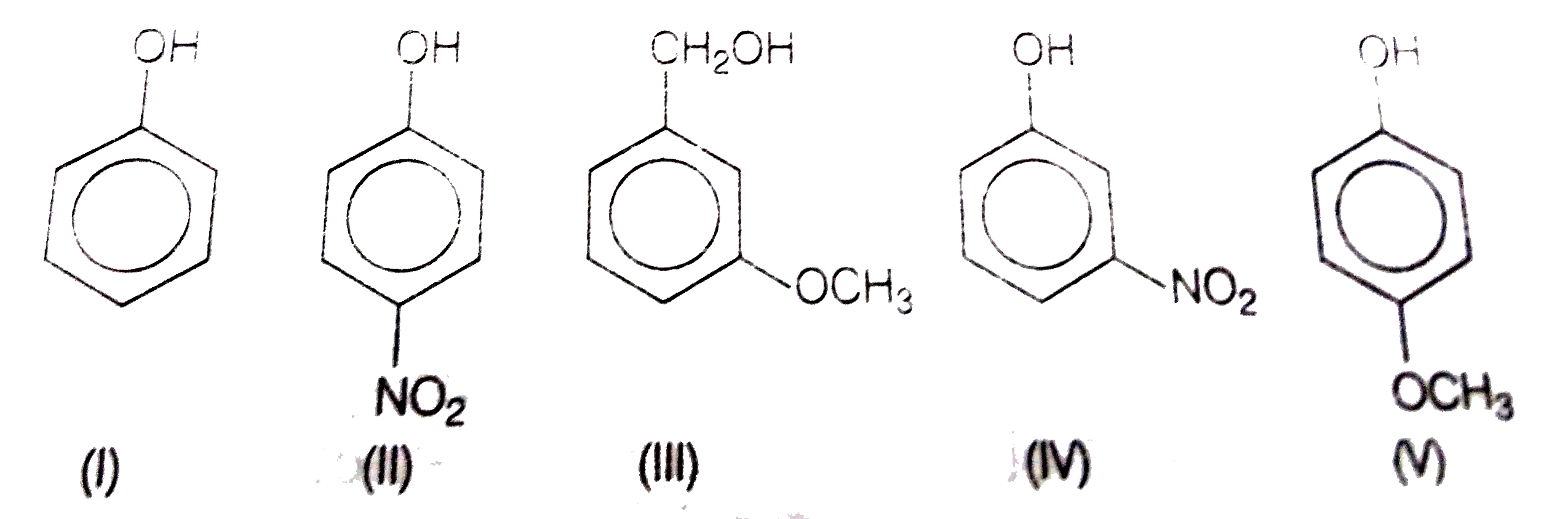
decreasing order of acidity of phenols
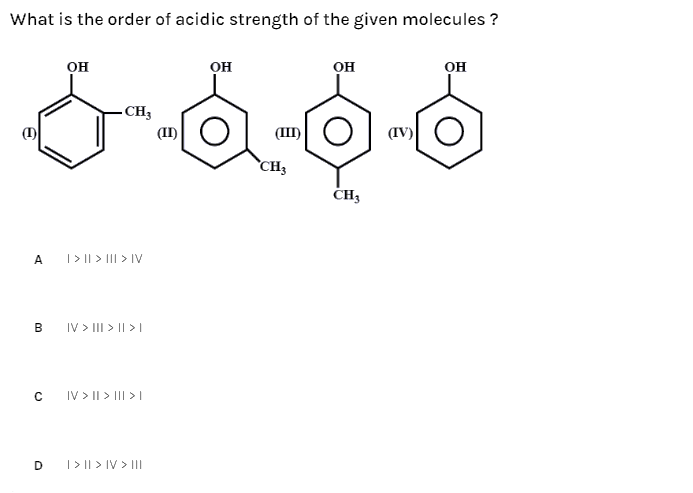
Which is the correct order of decreasing acidity?
Thus primary alcohols having least +I effect will be more acidic than secondary alcohols.
Similarly, secondary alcohols are more acidic than tertiary alcohols.
Therefore the order of decreasing acidity will be: CH3OH>C2H5OH>(CH3)2CHOH>(CH3)3COH.What is the correct order of acidity of phenols?
Therefore, The correct order of acidity is Para-nitrophenol Meta-nitrophenol Phenol Methyl phenol.
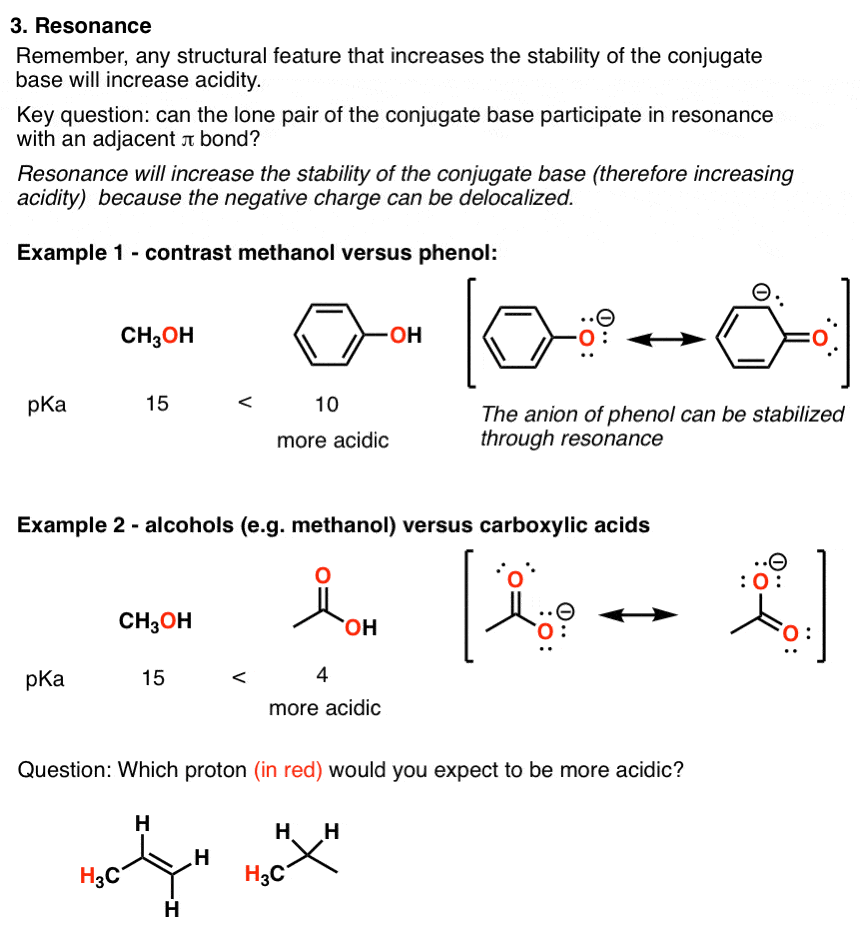
Electron releasing groups such as −CH3 decrease the acidic strength of phenol by destabilizing the phnoxide ion by increasing its negative charge while electron with drawing groups such as −NO2,−CN,and−CHO increase the acidic strength of phenol by stabilizing the phenoxide ion by dispersing its negative charge.
What is the decreasing order of acid strength of phenol?
The correct order of decreasing acid strength is b>d>a>c>e p-nitrophenol is most acidic and p-methoxy phenol is least acidic.
When an electron withdrawing group is para to OH group, the acidity is maximum.
When an electron releasing group is para to OH group, the acidity is minimum.9 jan. 2020
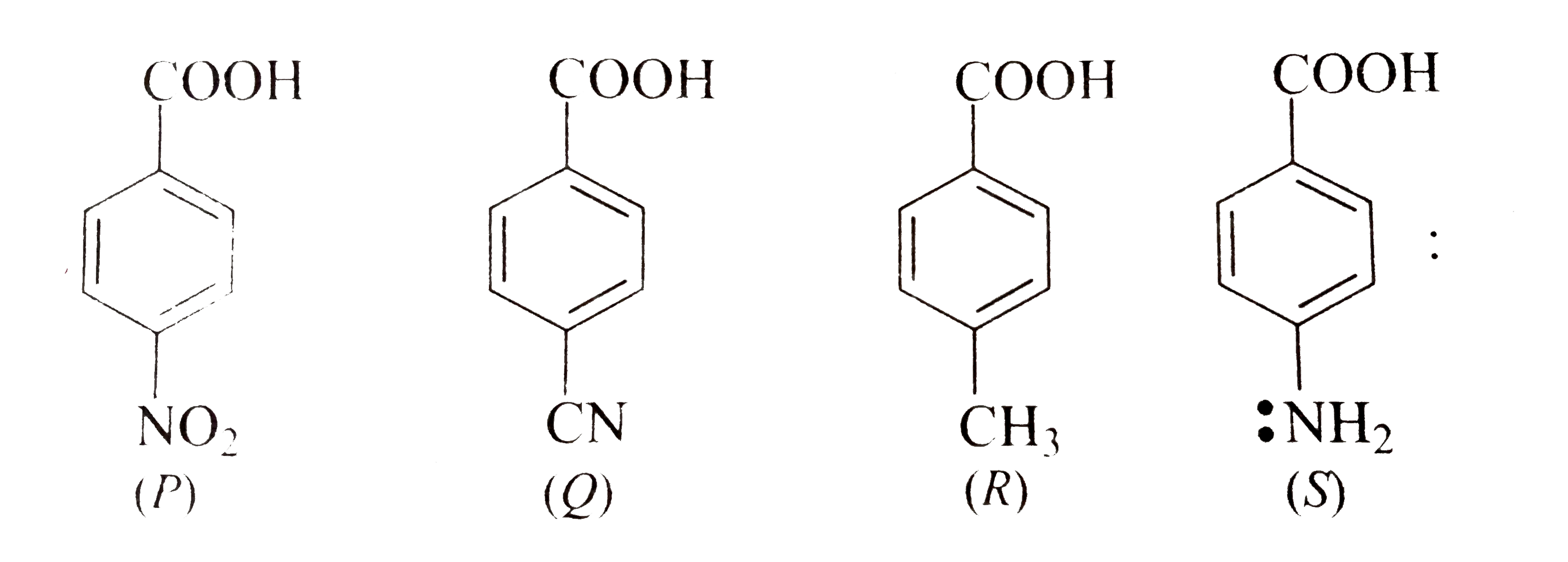
- Q > S > R > P is the order of acidity.
- Electron donating group decreases acidity.
- effect increases acidity.
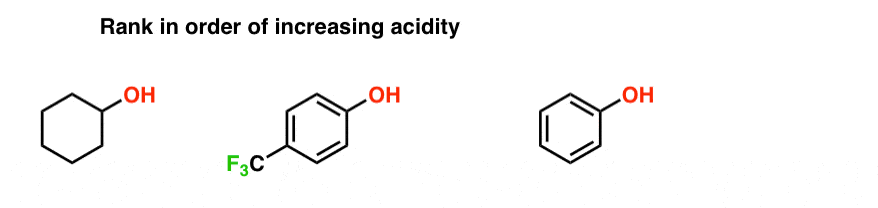
- This is due to the fact that the conjugate base of the acid, , is stabilised by delocalisation of the generated negative charge if group is electron-withdrawing.
|
Leep511.pdf
Apr 23 2018 Mark the correct order of decreasing acid strength of the ... Arrange water |
|
Alcohols Phenols and Ethers
decrease the polarity of O-H bond. This decreases the acid strength. For this reason the acid strength of alcohols decreases in the following order:. |
|
CHEMISTRY CL-12 MCQS TERM (2021-2022)
MR VENKATESH –PGT-CHEMISTRY KV STEEL PLANT (ALCOHOLS |
|
A Theoretical Investigation of Excited-State Acidity of Phenol and
experimental value. All the systems considered here are more acidic in the excited state with decreasing acidity variations in the order phenol |
|
A Study of the Adsorption of Aromatic Compounds Using Activated
as adsorbents for the removal of benzoic acid salicylic acid and phenol from acid > phenol. The descending order based on solubility from the adsorbate. |
|
Test2 ch17a Acid-Base Practice Problems
What is the pH of a 0.10 M solution of phenol? Which list has these acids in order of decreasing acid strength? a. LA > OA > MA d. OA > LA > MA. |
|
Chapter 5 Carboxylic Acids and Esters
Learn to recognize the carboxylic acid ester |
|
NCERT Exemplar Solutions For Class 12 Chemistry Chapter 11
(iii) Phenol. (iv) m-Chlorophenol. Solution: Option (iv) is the answer. 14. Mark the correct order of decreasing acid strength of the following compounds. |
|
Organic Chemistry II Exam 2
10 avr 2008 · 4 methyl-hexandic acid p-bromo benzoic acid methy 4-methyl Based on your Rank the following in decreasing order of acidity: 1 B most |
|
Acidity of phenols, effect of substituents on ac - Yengage
Thus phenols are more acidic than alcohols because resonance is impossible in order of decreasing acidity: phenol > m > p > o (least acidic) Working |
|
PHENOL - ResearchGate
UNIT- II: Phenols: Acidity of phenols, effect of substituents on acidity, qualitative tests, Structure and The decreasing order of acidity of nitrophenols is as |
|
Chapter 17: Alcohols and Phenols
substituents in alphabetical order 4 17 3: Properties of alcohols and phenols: acidity and basicity: reduces aldehydes, carboxylic acids, and esters |
|
NCERT Exemplar Problems Class 12 Chemistry Alcohols, Phenols
Mark the correct order of decreasing acid strength of the following Solution: -NO is an electron withdrawing group which increases the acidity of phenol and the |
|
Phenols
Electron withdrawing groups increases the acidity of phenol and the effect is more pronounced in ortho and para position compared to the meta position On the other hand, electron donating group tend to decreases the acidity of the phenols |
|
NCERT Exemplar Solutions For Class 12 Chemistry Chapter - Byjus
(i) Benzyl alcohol (ii) Cyclohexanol (iii) Phenol (iv) m-Chlorophenol Solution: Option (iv) is the answer 14 Mark the correct order of decreasing acid strength of |
|
Specimen Copy - Resonance DLP
Decreasing order of enol content for above carbonyl compounds is : 3 > 2 > 1 Groups which are – I, – m increases acidic character of phenol because |
|
OChem ACS Review 24 Phenols - Prexamscom
D II and IV 3 Arrange the following in order of decreasing acidity I benzoic acid (C6H5CO2H) II benzyl alcohol (C6H5CH2OH) III phenol (C6H5OH) |
|
Alcohols, Phenols and Ethers - NCERT
Alcohols, phenols and ethers are the basic compounds for the formation of In alcohols, the boiling points decrease with increase of branching in carbon chain Arrange the following compounds in increasing order of their acid strength: |