diels alder reaction mechanism pdf
|
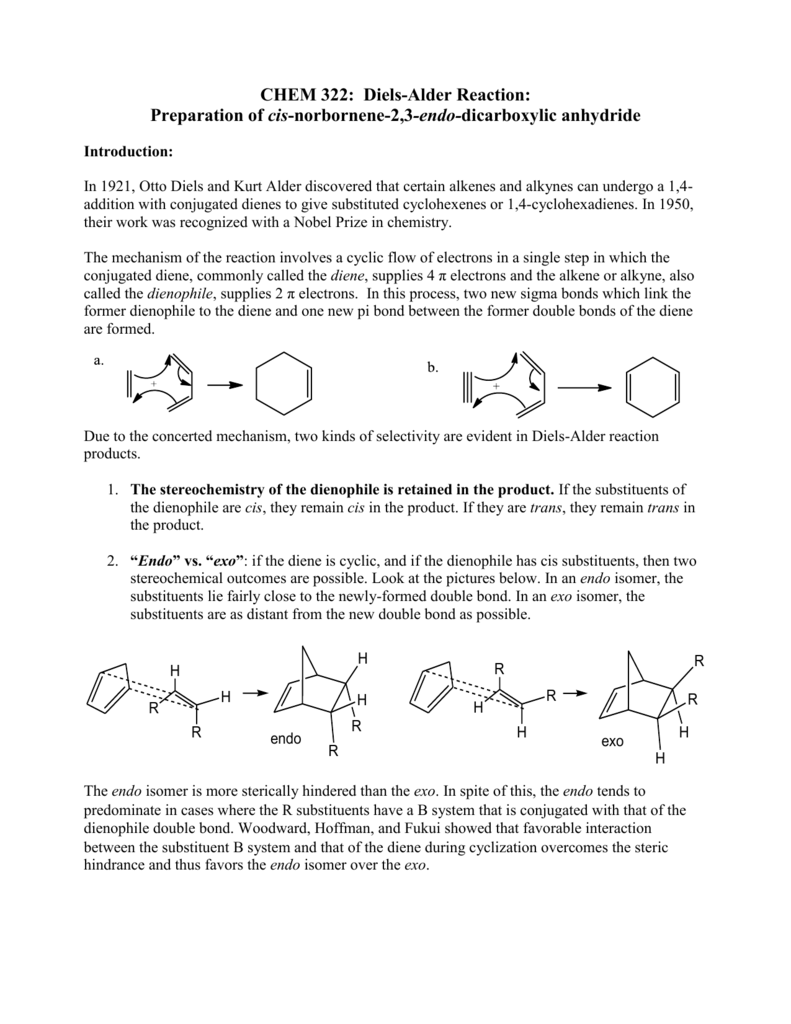
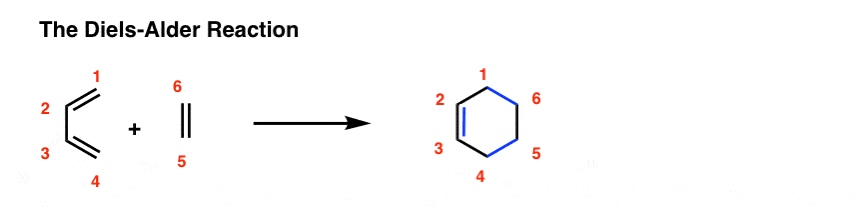
Diels-Alder Reaction
Mechanism R\' + R\' R\' concerted pericylic reaction with an aromatic transition state forms two new σ-bonds forms the endo product as shown by the HOMO-LUMO orbital interactions HOMO LUMO or LUMO HOMO Regioselectivity |
|
Diels-Alder Reaction
Mechanism: Pericyclic Reaction- proceeds in a single step via an \"aromatic\" transition state ‡ Diels-Alder Transition State = Benzene The diene must adopt an s-cis conformation to be reactive: s-cis (reactive conformation) s-trans (unreactive conformation) Endo vs Exo Transition State: Generally the endo transition state is favored |
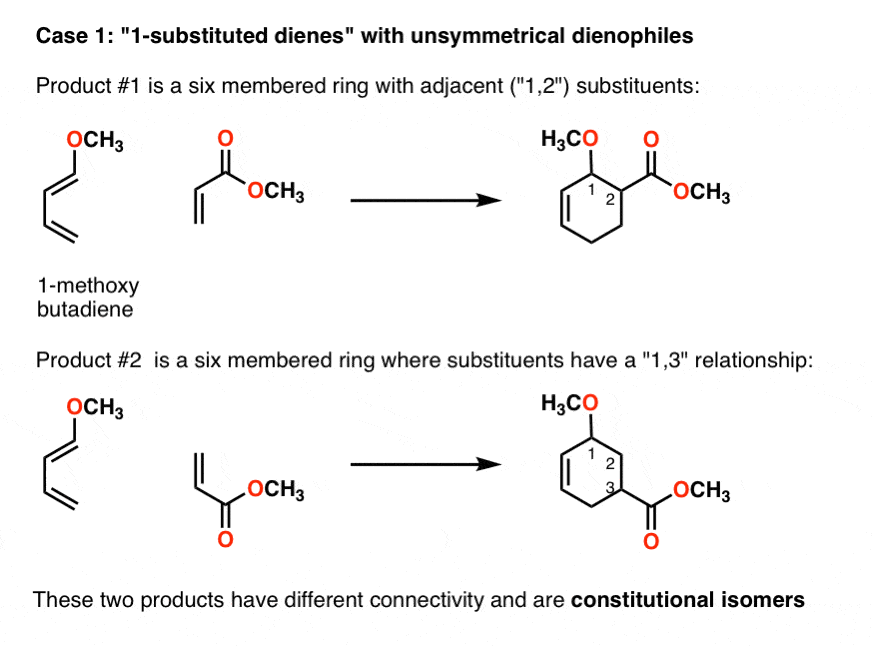
What is the regiochemistry of Diels-Alder reaction?
The regiochemistry of this Diels-Alder reaction is explained by looking at the dipolar resonance structures. The electron-rich carbon of the diene forms a bond with the electron-poor carbon of the dienophile.
Is Diels Alder click chemistry a novel route to graft copolymers?
Anthracene−maleimide-based Diels−Alder “click chemistry” as a novel route to graft copolymers Macromolecules, 39 ( 2006), pp. 5330 - 5336, 10.1021/ma060690c Multiarm star block copolymers via Diels–Alder click reaction J. Polym. Sci. A: Polym. Chem., 47 ( 2009), pp. 178 - 187, 10.1002/pola.23140
How does a Diels Alder reaction work?
Overlap between the highest occupied MO of the diene (HOMO) and the lowest unoccupied MO of the dienophile (LUMO) is thermally allowed in the Diels Alder Reaction, provided the orbitals are of similar energy. The reaction is facilitated by electron-withdrawing groups on the dienophile, since this will lower the energy of the LUMO.
Chemists Unanimously Agree: The Diels-Alder Reaction Is Awesome
But don’t take some random blogger’s word for it. Here are three eminent chemists opinion on this reaction. masterorganicchemistry.com
The Diels-Alder Reaction
OK. Are you ready to see it? Prepare yourself. Here it is: My work is done here, folks. Lesson over [drops mic] masterorganicchemistry.com
Don’T Be Underwhelmed
…wait. You’re not amazed? You’re not impressed? You are not blown away by the sheer power and beauty of this amazingly powerful process? You are forgiven. Truth be told, I was a little underwhelmed myself when learned this reaction back in undergrad. [Nor was I as impressed as I should have been with the Cope rearrangement, which, when drawn in its
The Basic Pattern of The Diels-Alder Reaction
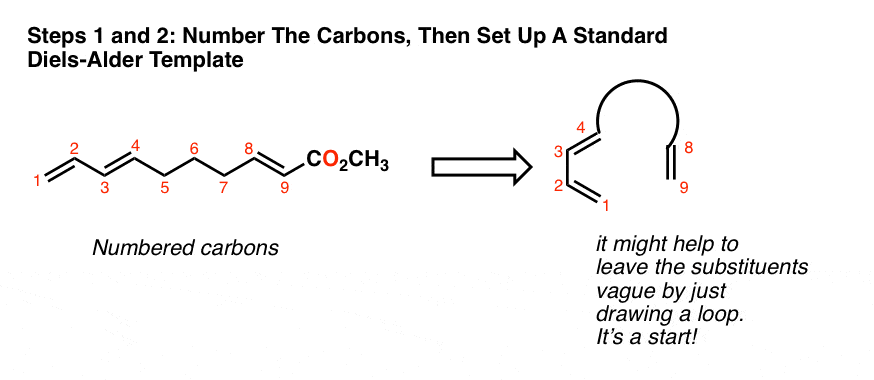
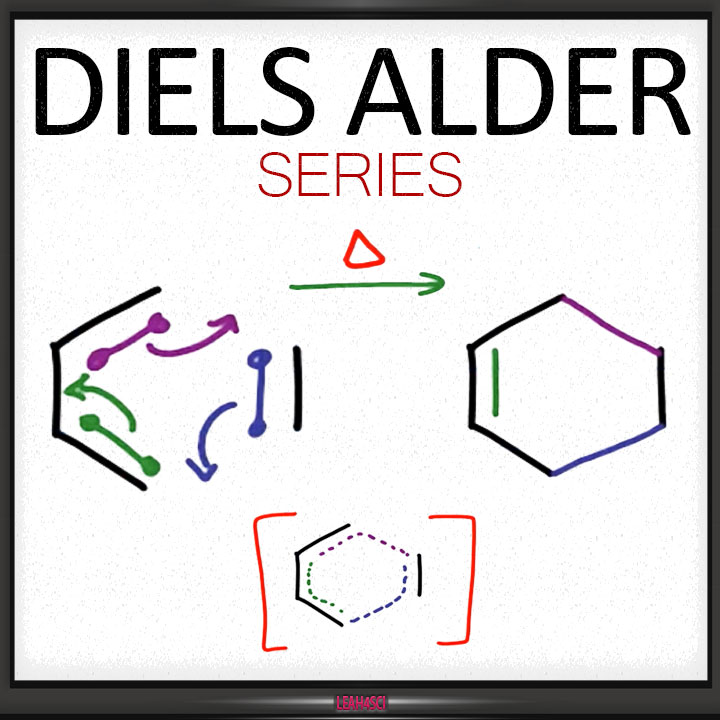
A Diels-Alder reaction brings together two components. 1. One part we call the “diene“, which is comprised of two adjacent (i.e. conjugated) pi bonds. 2. The second component is called the “dienophile“, which is to say “diene-loving”, and has at least one pi-bond. What bonds form, and what bonds break here? 1. Three pi bonds are broken. (C1-C2, C3-
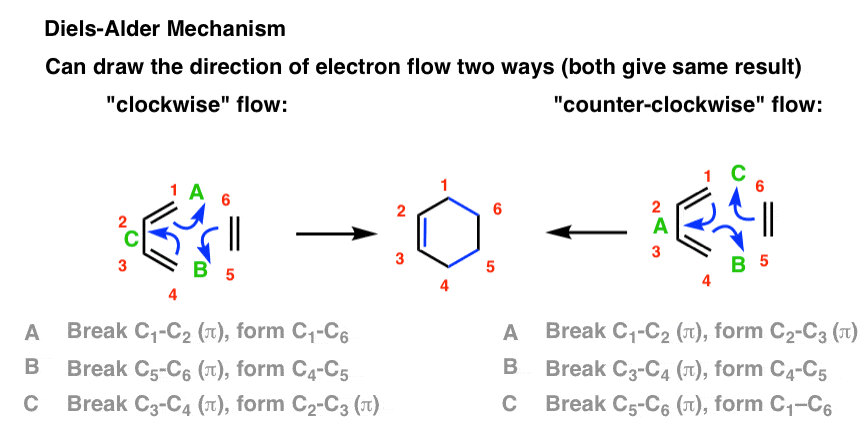
The Arrow-Pushing Mechanism of The Diels-Alder
My usual preference is to hold off on mechanisms until we’ve walked through some experimental facts, but here I will make an exception. There are two ways to draw the flow of electrons (both correct): clockwise and counter-clockwise. This flow of electrons is depicted with three arrows, which I’ve labelled A, B, and C. Pay attention what bonds form
Four Key Things to Know About The Diels-Alder Reaction
Having looked at the electron flow and the pattern of bonds that form and break, let’s continue with some very basic questions about the Diels-Alder. Here are four key points: 1. The Diene Must Be Conjugated 2. The Diene Must Be In The s-cisconformation 3. Substituents Do NOT Change the pattern of bonds formed/bonds broken… 4. … but they do affect
Four Key Things, Part 1: The Diene Must Be Conjugated
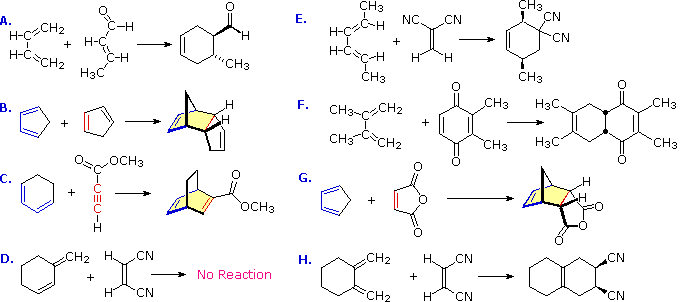
The diene mustbe conjugated to participate in a Diels-Alder reaction. No conjugation, no Diels-Alder. So while 1,3-butadiene readily undergoes the Diels-Alder reaction, 1,4 pentadiene (below) does not. (If you’re not clear about what constitutes a “conjugated” diene, you might want to visit this post.) masterorganicchemistry.com
Four Key Things, Part 2: The Diene Must Be in The s-cis Conformation
It’s not enough for the pi-bonds of the diene to be adjacent; the two C-C pi bonds must adopt a conformation where they are in the same plane (i.e. flat). But even that isn’t sufficient. The diene must adopt a conformation where the two pi-bonds are oriented cis to the central C-C single bond in order for the Diels-Alder to occur. In other words, t
Notes
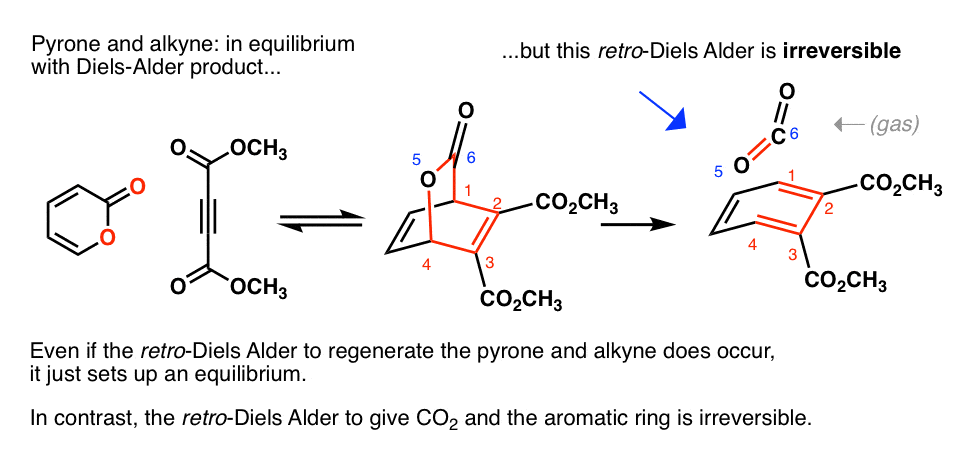
Note 1 For our purposes we’ll only discuss normal electron demand Diels Alder reactions, with an electron-rich diene and an electron-poor dienophile. However, there are examples of Diels-Alder reactions that proceed with electron-poor dienes and electron-rich dienophiles. These are known as inverse-electron demand Diels Alder reactions. Note 2– The
Appendix 2: Three Spectacular Examples of The Diels-Alder Reaction
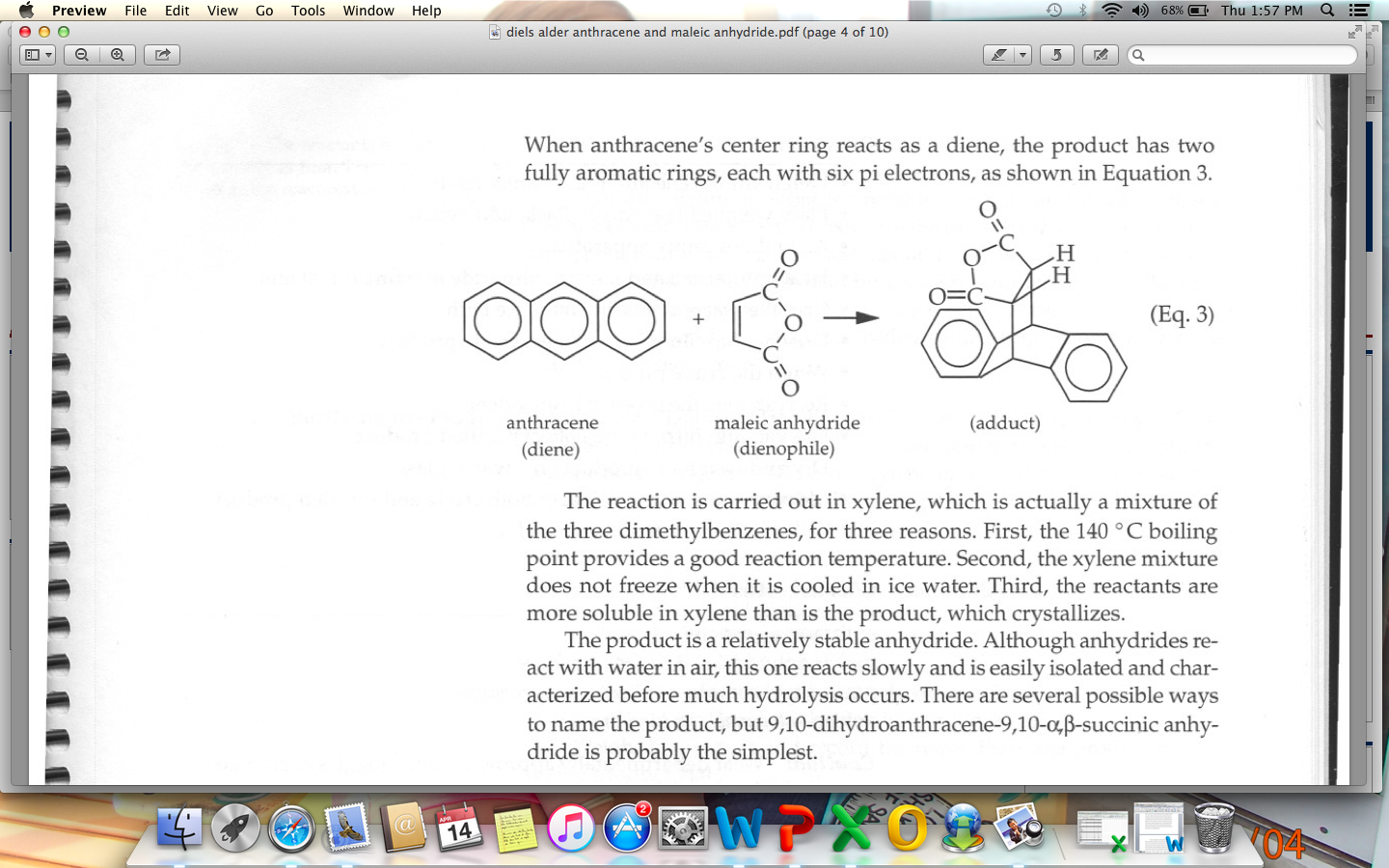
Advanced topic. There are countless examples of the Diels-Alder reaction being used for the syntheses of natural products. I picked three below. The examples will look complex to a beginner – and they are. However, the key point I want to hammer home is that the pattern of bonds that form and break in all of these reactions is NO DIFFERENT than the

Diels-Alder reaction Organic chemistry Khan Academy

Diels Alder Reaction

16.5 Diels-Alder Reactions Organic Chemistry
|
Diels-Alder Reaction (a very important reaction) Reaction between a
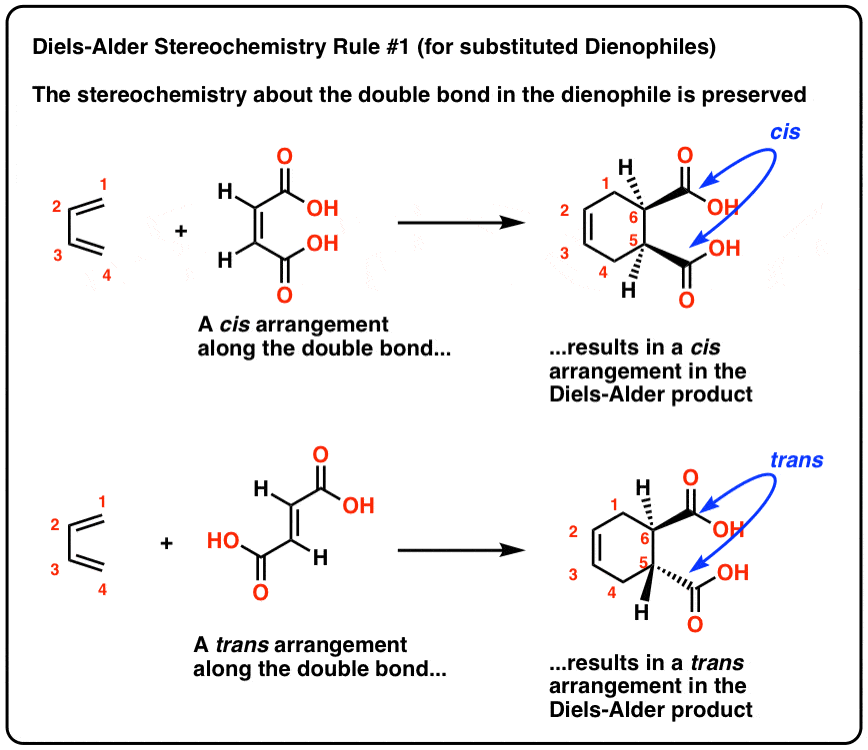
In the product the groups of the dienophile that are endo in the transition state will be cis to the groups on the outer rim of the diene (in the s-cis |
|
The Diels-Alder reaction
Diels-Alder reaction. • The 'cube' method is a nice way to visualise the relative stereochemistry. • Finally remember that the dienophile invariably reacts |
|
Efficient Computation of Free Energy Surfaces of Diels–Alder
Sep 28 2018 Diels–Alder Reactions in Explicit Solvent at Ab Initio ... cycloaddition) between a conjugated diene and a dienophile |
|
Diels-Alder Introduction
cycloaddition are calculated (the HOMO of the diene and the LUMO of the dienophile in a normal electron-demand Diels-Alder reaction) and orbitals of |
|
The Diels-Alder-Reaction with inverse-Electron-Demand a very
Dec 5 2009 Diels-Alder Reaction feature electron-rich diene and electron-poor dienophile compounds |
|
Trends in Diels Alder in Polymer Chemistry
Oct 5 2021 DA reaction is a simple and scalable toolbox. Though it is well-established that the furan/maleimide is the most studied diene/dienophile ... |
|
Experiment 13: The Diels-Alder Reaction of a Conjugated Diene in
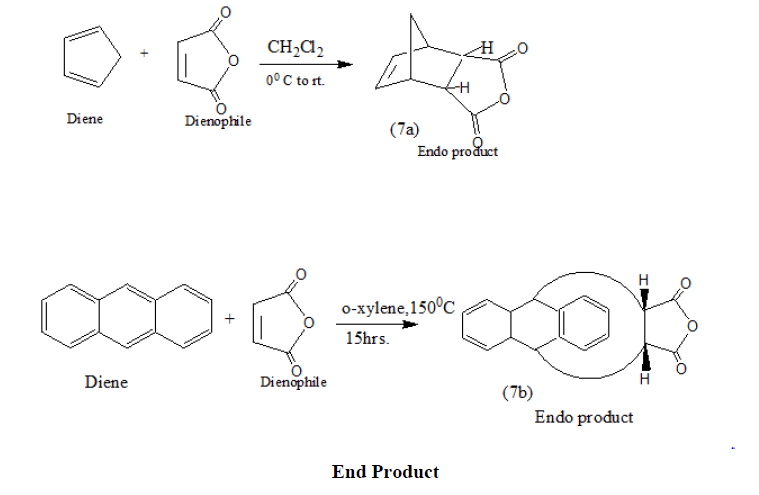
The addition of maleic anhydride to a diene yields entirely the endo product in which the bulkier parts of the dienophile are closer to the carbon-carbon double |
|
Diels–Alder Cycloaddition Reactions in Sustainable Media
Feb 15 2022 The Diels–Alder reaction |
|
The Diels-Alder Reaction: an Update Introduction
In this approach the sensitive diene or dienophile portions of the molecules are tied up with a convenient partner in a Diels-Alder reaction the. |
|
THE DIELS–ALDER REACTION WITH MOLECULAR OXYGEN AS
can be regarded as a photochemical Diels-Alder reaction in which oxygen acts as dienophile. It has recently been found that homoannular 13-dienes. |
|
The Diels-Alder reaction
If you are a little rusty on the Diels-Alder reaction either re-read your lecture notes or any standard organic text book draw a cube add the diene add dienophile |
|
Diels-Alder Reaction (a very important reaction) Reaction between a
In the product, the groups of the dienophile that are endo in the transition state will be cis to the groups on the outer rim of the diene (in the s-cis conformation) |
|
Extraordinary Mechanism of the Diels−Alder Reaction - SMU
19 jan 2016 · A first smaller charge transfer to the dienophile facilitates the rotation of gauche butadiene into its cis form The actual chemical processes are |
|
CYCLOADDITIONS IN ORGANIC SYNTHESIS
Although the Diels Alder reaction combines a diene and a dienophile to generate a cyclohexene, the reaction between simple 1,3- butadiene and ethene is very |
|
Diels-Alder reactions in synthesis and method - UiO - DUO
The Diels-Alder reaction in its simplest form Both the diene and the dienophile can be heavily substituted16 The mechanism has been investigated extensively |
|
The Diels-Alder Reaction - MIT
5 oct 2007 · construction, it was clear by 1970 that the Diels-Alder reaction would be Suprafacial with respect to the Dienophile Component H EWG RE |
|
Diels – Alder Reaction 1,4-CycloAddition Reaction of Dienes
4 Examples of Dienes and Dienophiles Prefer electron withdrawing groups on dienophile Prefer electron donating groups on diene |
|
The Diels-Alder Reaction: an Update Introduction
The reaction may be executed under relatively simple reaction conditions by heating together the two components, diene and dienophile, in non-polar solvents, |
|
UNIVERSITY OF CALIFORNIA, IRVINE Intramolecular Diels–Alder
IRVINE Intramolecular Diels–Alder Reactions in Organic Synthesis 7 Critical survey of the Diels–Alder reaction mechanism: Sauer, J ; Sustmann, R Angew |
|
34 - MSU chemistry
Predict the structure of the product of this Diels-‐Alder reaction CO2Me OMe Me3SiO + ? Solutions Manual to accompany Organic Chemistry 2e MeO2C H OMe So electronic factors dominate, perhaps because the dienophile has |















![Diels-Alder reactions II: The Reaction Mechanism - [PDF Document] Diels-Alder reactions II: The Reaction Mechanism - [PDF Document]](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/1-four-cases-of-diels-alder-reaction-unsymmetrical-diene-with-unsymmetrical-dienophile-gives-different-regioisomer-possibilities.gif)