dilution equation percentage
What does a more dilute solution mean?
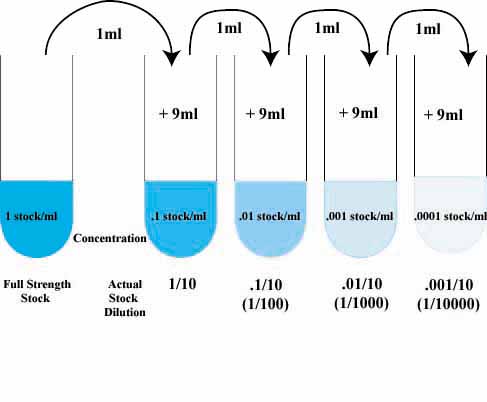
Well whenever you are trying to create a more dilute solution you would use that formula. A more dilute solution meaning a solution with a lower concentration than the original. You use this quite often in chemistry when you want to work with a solution with a specific concentration.
What is a dilution equation?
Concentrated chemicals often need to be diluted before use. The dilution equation allows for the dilution of a stock solution into a working solution. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). Based on which is selected, a 10% solution can be made.
Does adding a stock solution affect the concentration of a diluted solution?
Of course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes. Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions.
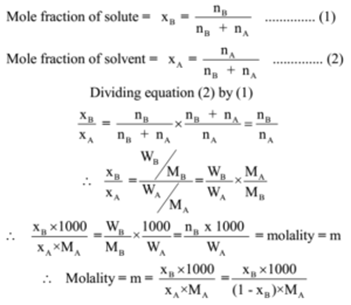
How do you calculate concentration after a dilution?
You dilute a solution whenever you add solvent to a solution. Adding solvent results in a solution of lower concentration. You can calculate the concentration of a solution following a dilution by applying this equation: where M is molarity, V is volume, and the subscripts i and f refer to the initial and final values.
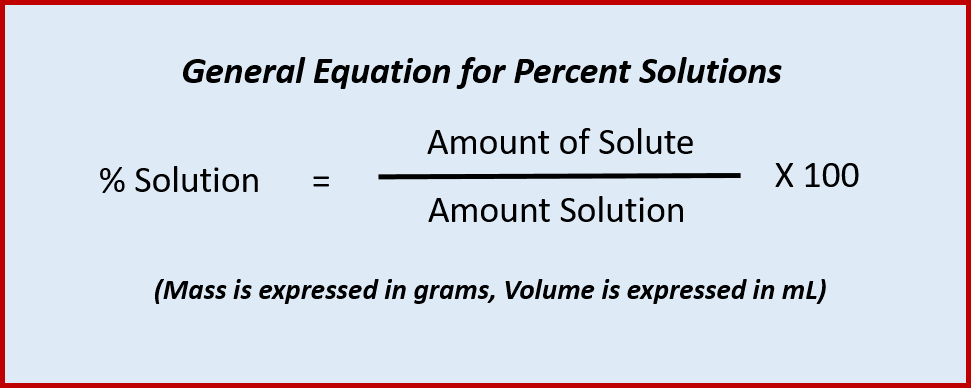
Percent Composition by Mass
This is the mass of the solute divided by the mass of the solution (mass of solute plus mass of solvent), multiplied by 100.Example: Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt.Solution: 20 g NaCl / 100 g solution x 100 = 20% NaCl solution thoughtco.com
Volume Percent
Volume percent or volume/volume percent most often is used when preparing solutions of liquids. Volume percent is defined as: v/v % = [(volume of solute)/(volume of solution)] x 100% Note that volume percent is relative to the volume of the solution, not the volume of solvent. For example, wine is about 12% v/v ethanol. This means there is 12 ml et
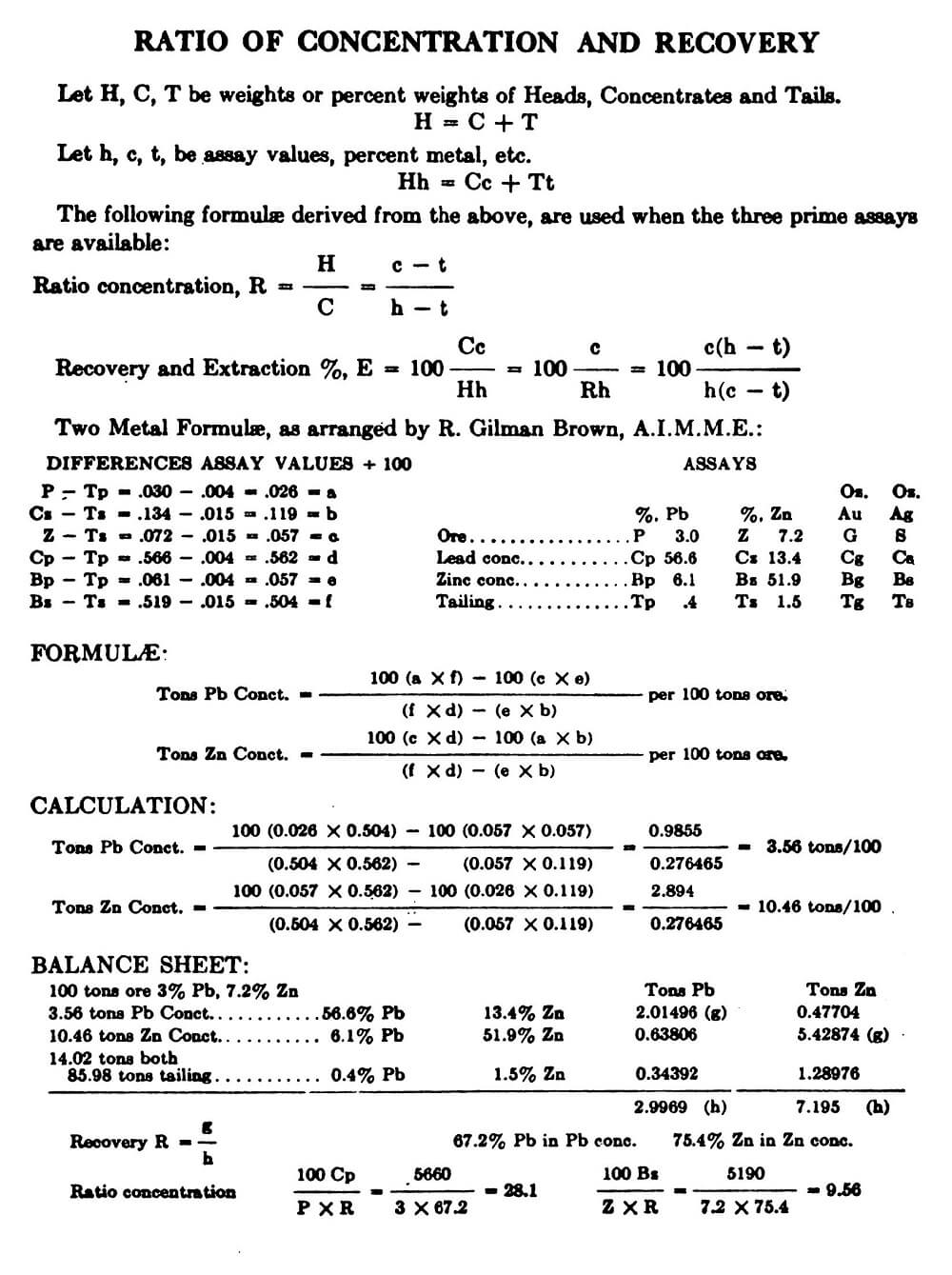
Mole Fraction
This is the number of moles of a compound divided by the total number of moles of all chemical species in the solution. Keep in mind, the sum of all mole fractions in a solution always equals 1.Example:What are the mole fractions of the components of the solution formed when 92 g glycerol is mixed with 90 g water? (molecular weight water = 18; mol
Molarity
Molarity is probably the most commonly used unit of concentration. It is the number of moles of solute per liter of solution (not necessarily the same as the volume of solvent).Example: What is the molarity of a solution made when water is added to 11 g CaCl2 to make 100 mL of solution? (The molecular weight of CaCl2 = 110)Solution: 11 g CaCl2 / (
Molality
Molality is the number of moles of solute per kilogram of solvent. Because the density of water at 25°C is about 1 kilogram per liter, molality is approximately equal to molarity for dilute aqueous solutions at this temperature. This is a useful approximation, but remember that it is only an approximation and doesn't apply when the solution is at a
Normality
Normality is equal to the gram equivalent weight of a solute per liter of solution. A gram equivalent weight or equivalent is a measure of the reactive capacity of a given molecule. Normality is the only concentration unit that is reaction dependent.Example: 1 M sulfuric acid (H2SO4) is 2 N for acid-base reactions because each mole of sulfuric acid
|
PREPARING SOLUTIONS AND MAKING DILUTIONS
below is a quick approach to calculating such dilutions where: mixed as dry mass (g) per volume where #g/100 ml = percent concentration. A 10% NaCl. |
|
Dilution of solutions for nurses
Similarly for a percentage stock strength solution the equation will be as in the following example. Example Calculate the amount of. (i) stock solution |
|
Laboratory Math II: Solutions and Dilutions
For example: NaCl in solution consists of positive charged sodium ions and negatively charged chloride ions. What is relevant is solute particles” per unit |
|
Medical Calculations
Performing the proportion equation by cross multiplication: Example: to convert 500 mg to g Dilution: reduction of a concentration of a substance. |
|
Pharmaceutical calculation
16-Jul-2021 If a mixture of a given percentage or ratio strength is diluted to twice its original quantity its active ingredient will be contained in twice ... |
|
Mathcentre
Calculating how much base to add to a product to achieve a lower desired concentration. percentages cancel out. Student Learning Advisory Service. |
|
1st International Standard 2019 for HCT 15 Cancer Genome
16-Mar-2020 consensus variant percentage for PIK3CA c.1633G>A (E545K) and ... dilution formula based on the variant and total gene copy number per. |
|
Prediction and Measurement of Weld Dilution in Robotic CO Arc
as well as the gas mixture on dilution percentage in robotic arc welding. simply (+) and (-) respectively according to the equation. |
|
Beers Law: Determining the Concentration of a Solution
and the volumes used to make the dilution to calculate the concentration of the original unknown solution. Use the following equation:. |
|
PREPARING SOLUTIONS AND MAKING DILUTIONS
Many reagents are mixed as percent concentrations When working with a dry chemical it is mixed as dry mass (g) per volume where #g/100 ml = percent concentration A 10 NaCl solution is equal to 10 g dissolved in 100 ml of solvent |
|
Concentrations and Dilutions INTRODUCTION
Calculate volume/volume percent concentrations • Calculate dilutions of stock solutions INTRODUCTION Concentrations of many pharmaceutical preparations |
|
Dilution and Concentration
dilution and concentration uncomplicates these problems Many problems Calculating the percentage or ratio strength of a solution made by diluting or con- |
|
Dilution of solutions for nurses - Mathcentre
Similarly for a percentage stock strength solution the equation will be as in the following example Example Calculate the amount of (i) stock solution required, and |
|
Diluting a % solution - Mathcentre
Calculating how much base to add to a product to achieve a lower desired concentration Example percentages cancel out Student Learning Advisory Service |
|
Calculating Liquid Chemical Dilutions - RPC-Rabrenco
be complied with when working with chemicals Example No 1 - Dry Powder- weight Dilutions Assume desired concentration of citric acid solution is 15 percent |
|
6 Percentage, Ratio Strength, and Other Expressionsppt [相容模式]
for calculating percentage weight-in-volume (e g , 1 w/v = 1 of [100 mL taken to be] 100 g = 1 g in 100 mL) Weight of Active Ingredient in a Specific Volume, |
|
How to Make Simple Solutions and Dilutions
The formula belorv is a quick approach to calculating such dilutions Ib conrert ftom solution to molerity, multiply the solution by l0 to exprcss the percent |
|
Solution Dilution Equation - Free eBooks in the Genres you Love
oz) imperial gallon, liquid (gal) imperial pint (pt) litre Dilution Calculator - for percent solutions Solution:20 g NaCl / 100 g solution x 100 = 20 NaCl solution |