distribution of molecules with velocity is represented by the curve
How do you calculate the speed of a molecule?
Although it is impossible to determine the speeds of individual molecules, it is possible to calculate the distribution of velocities over different fractions of molecules using probability considerations. This distribution is called the Maxwell-Boltzmann distribution.
Why is the distribution of molecular speeds important?
The actual distribution of speeds has several interesting implications for other areas of physics, as we will see in later chapters. The motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules has a predictable distribution of molecular speeds.
What is the Maxwell-Boltzmann distribution of molecular speeds?
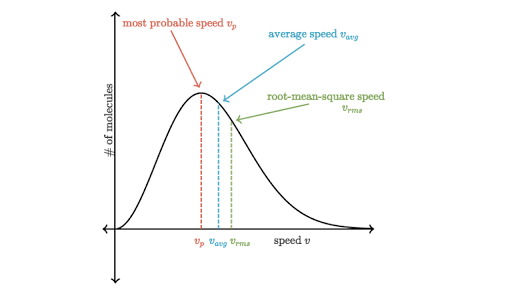
To understand this figure, we must define a distribution function of molecular speeds, since with a finite number of molecules, the probability that a molecule will have exactly a given speed is 0. Figure 2.15 The Maxwell-Boltzmann distribution of molecular speeds in an ideal gas. The most likely speed v p is less than the rms speed v rms.
Where is the most probable speed in a distribution curve?
As stated above, C mp is the most probable speed, thus it will be at the top of the distribution curve. To the right of the most probable speed will be the average speed, followed by the root-mean-square speed.
Overview
In a gas, there are lots of molecules traveling at lots of different speeds. Here's a framework for thinking about that. What is the Maxwell-Boltzmann distribution? The air molecules surrounding us are not all traveling at the same speed, even if the air is all at a single temperature. Some of the air molecules will be moving extremely fast, some will be moving with moderate speeds, and some of the air molecules will hardly be moving at all. Because of this, we can't ask questions like "What is the speed of an air molecule in a gas?" since a molecule in a gas could have any one of a huge number of possible speeds. So instead of asking about any one particular gas molecule, we ask questions like, "What is the distribution of speeds in a gas at a certain temperature?" In the mid to late 1800s, James Clerk Maxwell and Ludwig Boltzmann figured out the answer to this question. Their result is referred to as the Maxwell-Boltzmann distribution , because it shows how the speeds of molecules are distributed for an ideal gas. The Maxwell-Boltzmann distribution is often represented with the following graph. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region, it means that there are more gas molecules moving with those speeds. [Wait, isn't the probability equal to zero for a gas molecule to be moving at any exact speed?] khanacademy.org
What is the Maxwell-Boltzmann distribution?
The air molecules surrounding us are not all traveling at the same speed, even if the air is all at a single temperature. Some of the air molecules will be moving extremely fast, some will be moving with moderate speeds, and some of the air molecules will hardly be moving at all. Because of this, we can't ask questions like "What is the speed of an air molecule in a gas?" since a molecule in a gas could have any one of a huge number of possible speeds. So instead of asking about any one particular gas molecule, we ask questions like, "What is the distribution of speeds in a gas at a certain temperature?" In the mid to late 1800s, James Clerk Maxwell and Ludwig Boltzmann figured out the answer to this question. Their result is referred to as the Maxwell-Boltzmann distribution , because it shows how the speeds of molecules are distributed for an ideal gas. The Maxwell-Boltzmann distribution is often represented with the following graph. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region, it means that there are more gas molecules moving with those speeds. [Wait, isn't the probability equal to zero for a gas molecule to be moving at any exact speed?] Notice that the graph is not symmetrical. There is a longer "tail" on the high speed right end of the graph. The graph continues to the right to extremely large speeds, but to the left the graph must end at zero (since a molecule can't have a speed less than zero). The actual mathematical equation for the Maxwell-Boltzmann distribution is a little intimidating and not typically needed for many introductory algebra classes. khanacademy.org
What does root-mean-square speed mean?
You might think that the speed located directly under the peak of the Maxwell-Boltzmann graph is the average speed of a molecule in the gas, but that's not true. The speed located directly under the peak is the most probable speed vp , since it is the speed that is most likely to be found for a molecule in a gas. The average speed vavg of a molecule in the gas is actually located a bit to the right of the peak. The reason the average speed is located to the right of the peak is due to the longer "tail" on the right side of the Maxwell-Boltzmann distribution graph. This longer tail pulls the average speed slightly to the right of the peak of the graph. Another useful quantity is known as the root-mean-square speed vrms . This quantity is interesting because the definition is hidden in the name itself. The root-mean-square speed is the square root of the mean of the squares of the velocities. Mean is just another word for average here. We can write the root-mean-square speed mathematically as, vrms=1N(v12+v22+v32+…) It might seem like this technique of finding an average value is unnecessarily complicated since we squared all the velocities, only to later take a square root. You might wonder, ""Why not just take an average of the velocities?" But remember that velocity is a vector and has a direction. The average gas molecule velocity is zero, since there are just as many gas molecules going right (+ velocity) as there are going left (- velocity). This is why we square the velocities first, making them all positive. This ensures that taking the mean (i.e. average value) will not give us zero. Physicists use this trick often to find average values over quantities that can take positive and negative values (e.g. voltage and current in an alternating current circuit). [Is there a reason we bother to talk about root-mean-square speeds?] khanacademy.org
What does the area under a Maxwell-Boltzmann distribution represent?
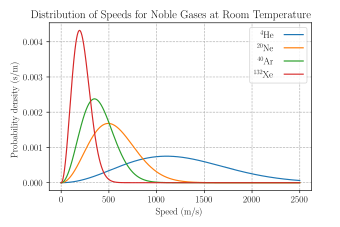
The y-axis of the Maxwell-Boltzmann distribution graph gives the number of molecules per unit speed. The total area under the entire curve is equal to the total number of molecules in the gas. If we heat the gas to a higher temperature, the peak of the graph will shift to the right (since the average molecular speed will increase). As the graph shifts to the right, the height of the graph has to decrease in order to maintain the same total area under the curve. Similarly, as a gas cools to a lower temperature, the peak of the graph shifts to the left. As the graph shifts to the left, the height of the graph has to increase in order to maintain the same area under the curve. This can be seen in the curves below which represent a sample of gas (with a constant amount of molecules) at different temperatures. As the gas gets colder, the graph becomes taller and more narrow. Similarly, as the gas gets hotter the graph becomes shorter and wider. This is required for the area under the curve (i.e. total number of molecules) to stay constant. If molecules enter the sample, the total area under the curve would increase. Similarly, if molecules were to leave the sample, the total area under the curve would decrease. khanacademy.org
Example 1: Cooling a gas
A gas of diatomic nitrogen is in a sealed container. The sealed container is then placed in an ice bath and reaches a lower equilibrium temperature with the ice bath. What happens to the following quantities as the gas cools? (select two correct statements) Choose all answers that apply: Choose all answers that apply: •(Choice A) The root-mean-square molecular speed of the gas increases khanacademy.org
Example 2: Change in the gas
A gas has a distribution of speeds that looks like the following. Which one of the following series of actions could cause the distribution graph to change from curve 1 to curve 2, as seen below? Choose 1 answer: Choose 1 answer: •(Choice A) Hotter gas molecules are allowed to enter and come into equilibrium with the initial gas. khanacademy.org
Want to join the conversation?
Log in khanacademy.org

Distribution of molecules with velocity is represented by the curveVelocity corresponding to poi

Kinetic Theory 03 : RMS Velocity Maxwells distribution of Velocities and Mean Free Path

Maxwells velocity distribution Law Derivation Kinetic Theory of Gases Lecture 2
|
Maxwells Distribution Law for the distribution of molecular speeds
Generally gas molecules have velocity components in all three directions (vx |
|
Scaling Up: Molecular to Meteorological via Symmetry Breaking and
24 feb. 2022 molecular velocity vectors after 9.9 collision times whereas the thinner arrows represent simulation by the Navier–Stokes equation. |
|
Impact of a hydrophobic ion on the early stage of atmospheric
5 nov. 2019 velocity distribution of the water molecules evaporated from excited ... sulfuric acid and water was shown to increase significantly the. |
|
Molecular dynamics study on characteristics of reflection and
16 mar. 2021 The kinetic boundary condition (KBC) represents the evaporation or ... the velocity distribution function of gas/vapor molecules. |
|
CLASS XI
speeds shown in the plot is called Maxwell-Boltzmann distribution of Maximum in the curve represents speed possessed by maximum number of molecules. |
|
BASICS OF MOLECULAR BEAM-SURFACE SCATTERING
time-of-flight (TOF) distributions an example of which is shown in Figure molecules on the surface is proportional to the perpendicular velocity of the ... |
|
Maxwell distribution law of Velocity - Shailaj Kumar Shrivastava.pdf
The distribution of molecular velocity the velocity point for the given molecule. ... value of Curve (Cm) represents relocity possesses by the. |
|
Molecular Dynamics Simulations of Shockwave Affected STMV Virus
18 mar. 2022 water molecules at the edge of the box with an elevated velocity ... single shockwave pulses—each curve represents the time evolution of the. |
|
Computational Tool - Molecular Dynamics Simulations of Molecules
7 may. 2019 (c) Velocity probability density function obtained from a linear fit of the displace- ment curves per molecule (light blue in a) is shown; the ... |
|
Numerical Simulation of the Photobleaching Process in Laser
20 dic. 2021 velocity vector D is the diffusivity of the dye molecule in the solution |
|
Additional content in Chemistry based on Revised syllabus - NCERT
Also, speed distribution curve broadens at higher temperature hydrogen chloride in 10 mol of water can be represented by the following equation |
|
Maxwell Boltzmann WS Keypdf
8) Which temperature has the most molecules with speeds above 600 m/s? Dotted - 600 a) Sketch the new shape of the distribution curve b) Indicate the new |
|
Some Properties of the Maxwell-Boltzman (M-B) Velocity Distribution
motion with velocity v, the microscopic velocity distribution depends on the orientation tive) ion (j = 0 is the ion ground state), gk and gj represent the degeneracies two similar curves with respect to G (hyperbola in the particular case of Chemistry plasma, 9 Closure hydrodynamic equation, 241 transport equation, 240 |
|
Chapter 2 KINETIC THEORY
These parameters are macrsocopic averages over the distribution of particle velocities and/or positions Show that low order velocity moments of the Boltzmann equation give The left hand side terms represent the change of energy density with time and the energy flow (c) the average kinetic energy of the molecules |
|
CH1101 General and Physical Chemistry 2012 Basic
Gas molecules represented as point masses: When gas molecule collides ( with speed vx) with wall of MB Velocity Distribution Curves : Effect of Molar Mass |
|
Lecture 2-3
Gas molecules represented as point p p When gas molecule collides (with speed vx) with wall of MB Velocity Distribution Curves : Effect of Molar Mass |
|
Maxwells Distribution Law for the distribution of molecular speeds
Boltzmann distribution law and the classical formula for the translational At any given moment the velocity of each molecule is represented by a dot Near the origin, curves are parabolic because of the dominance of the v2 term in the |
|
On the Dynamical Theory of Gases - JSTOR
gravity of the molecules, and whose magnitude is represented very nearly by a plane curve about this centre of gravity, and the two curves will be similar to each distribution of velocity such that the number of molecules whose velocity lies |
|
CHM 231 LECTURE NOTE COURSE SYNOPSIS
The distribution of molecular velocities Let V represent the volume of the cubic box, then V=a 3 ∴ V 2 (i) The smaller molecules like H2 and He, the curve |