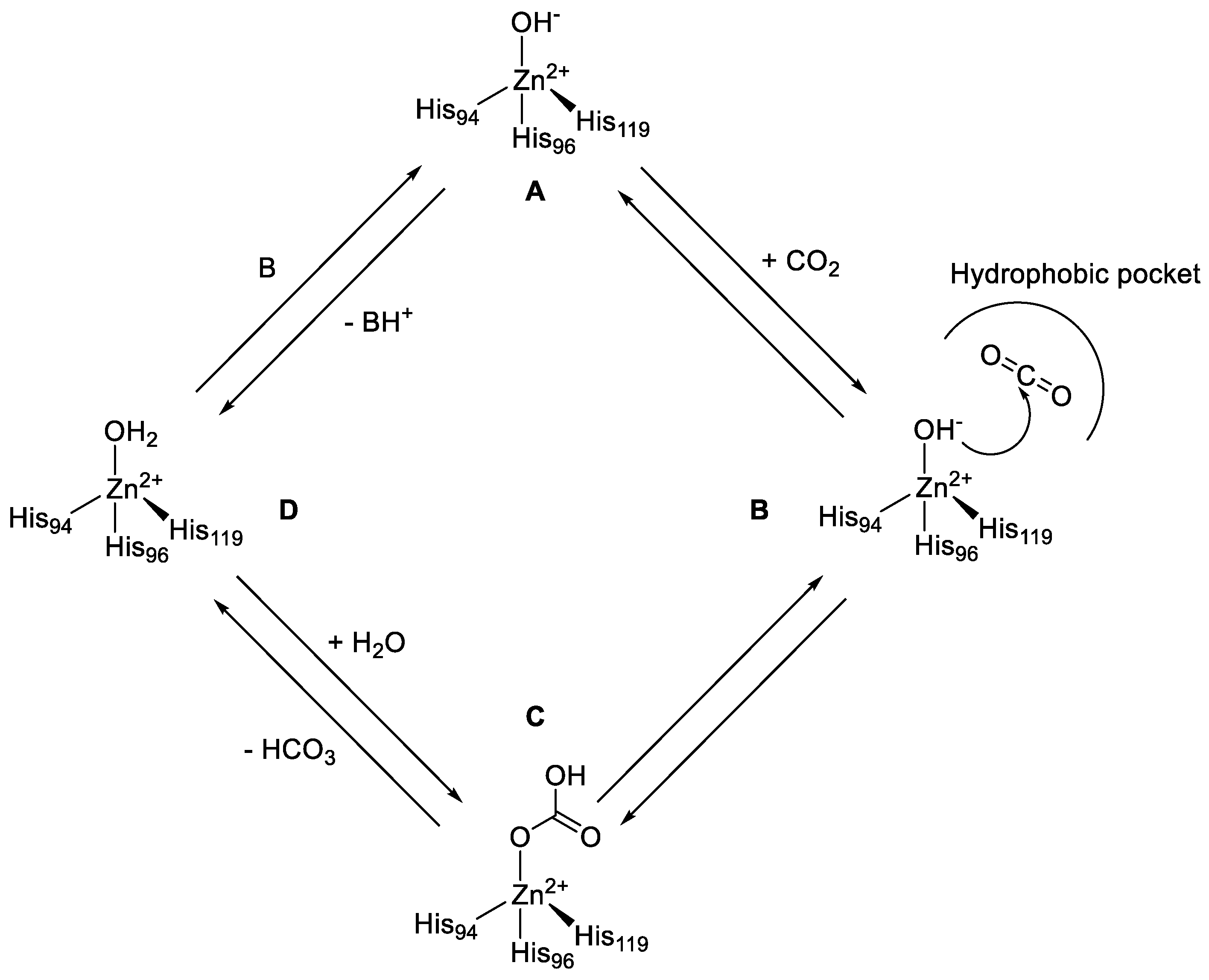
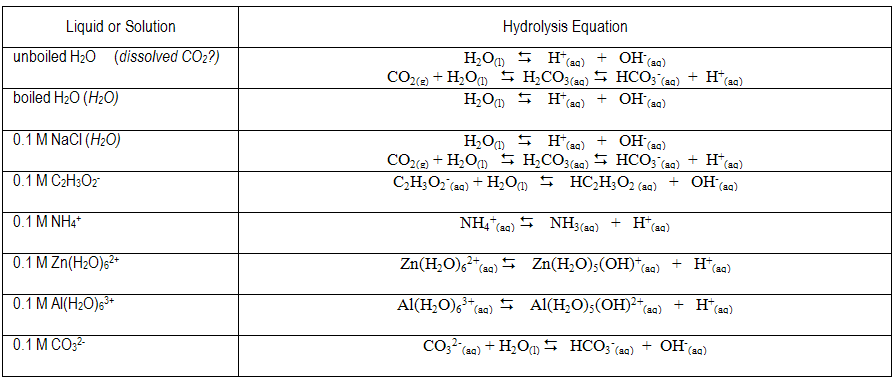
hco3 hydrolysis equation
|
Hydrolysis: Examples:
Eg. Find the predominant hydrolysis of the hydrogen carbonate ion (HCO3. -) and write the net-ionic equation for it. To find the Ka of HCO3. |
|
NaHCO3 + H2O Na+ + HCO3 (100%ionization) HCO3 + H2O CO3 +
For example consider sodium bicarbonate dissolved in water. B. Mass-balance equations are equations that relate the equilibrium concentrations of ... |
|
Exp 17 REACTIONS OF SALTS WITH WATER F 08
equilibrium constant of the hydrolysis reaction. Setting the K-expression in the Will the solution of such a salt be acidic due to the reaction: HCO3. |
|
The Ionization Constant of HCO3- from 0 to 50°
left of this equation it is necessary to correct for hydrolysis |
|
911 Metallurgist
-) > Ka (HCO3. -) the ion HCO3. - predominantly undergoes BASE HYDROLYSIS. ( 2.3 x 10-8 ) (5.6 x 10-11 ). And the net-ionic equation for the predominant |
|
Sec 4.13 – Hydrolysis (notes)
spectators are eliminated in net ionic equations for hydrolysis! And the net-ionic equation for the predominant hydrolysis is: HCO3. |
|
Solubility of Calcium Carbonate The solubility of salts of weak acids
HCO3. -. + OH. -. The hydrolysis decreases the concentration of CO3. 2- which pulls the solubility equilibrium to the right making CaCO3 more soluble. |
|
WEAK ACIDS AND BASES
5) Now write an equation for the hydrolysis reaction by the salt. HCO3. —. º H+. + CO3. 2—. K2 = 4.8 x 10—11. Overall: H2CO3. º 2 H+. + CO3. |
|
On the Stability of Uranium Carbide in Aqueous Solution—Effects of
10 sept. 2021 commonly occurring groundwater constituent HCO3. ? facili- ... hydrolysis reaction is often generally described as. UC. 2H O. UO. CH. 2. 2. |
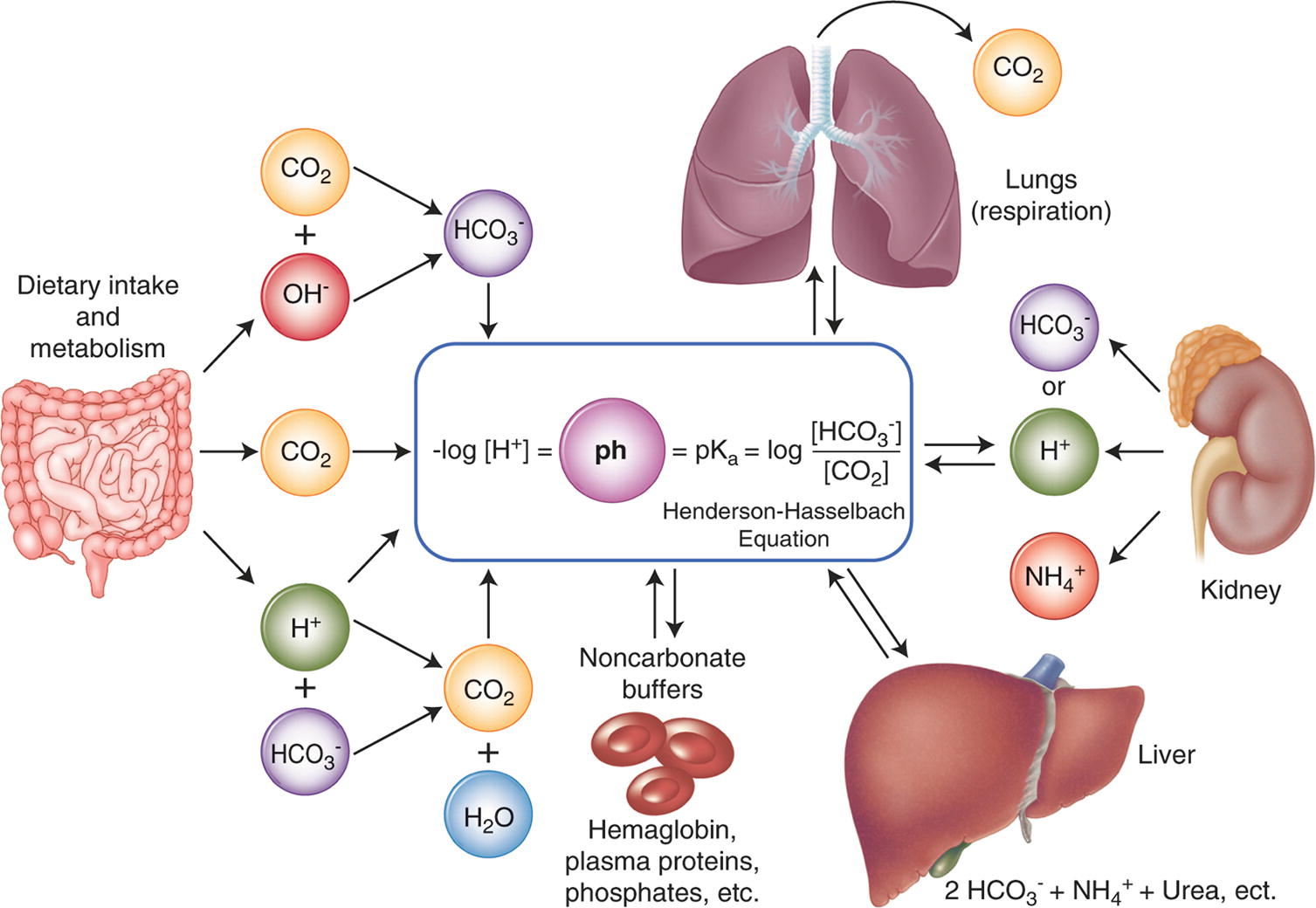
| Lesson 3: Ocean Acidification |
|
Hydrolysis: 911 Metallurgist
Hydrolysis: - Reaction between a salt (ion or ions in a salt) and water to produce an acidic or basic solution - Net ionic equations for hydrolysis: |
|
Exp 17 REACTIONS OF SALTS WITH WATER F 08
Hydrolysis as applied to water solutions of inorganic compounds can be defined as the reaction of water with one or both ions of a salt to form a weak acid |
|
The Ionization Constant of HCO3- from 0 to 50°
determine the ionization constant of HCO3- at 25 and 38° with the thermodynamic equation for the reaction Maclnnes and Belcher make the hydrolysis |
|
Sec 413 – Hydrolysis (notes) - Arcuric Acid
predominantly undergoes BASE HYDROLYSIS And the net-ionic equation for the predominant hydrolysis is: HCO3 - (aq) + H2O( |
|
HYDROLYSIS
The hydrolysis of halogenated hydrocarbons leads to alcohols (or poly alcohols which rapidly equilibrate to corresponding carbonyl compounds) The reaction |
|
Speciation studies in aqueous HCO3 –CO3 solutions A - CiteSeerX
carbonate (NaHCO3) were purchased from Merck Darmstadt (pro analysi >99 5 ) hydrolysis reaction as a function of concentration and temperature |
|
On the hydration and hydrolysis of carbon dioxide - Berkeley
24 août 2011 · Because the relevant reaction rates are not known it is not possible to reliably estimate the adjusted composition when the liquid microjet is |
|
Chapter 16 Acid-Base Equilibria • Acids and bases are found in
Write a balanced equation showing how the following substances behave as acids in water and identify the conjugate acid-base pairs • HNO3 HCO3 - H3PO4 |
|
E20 HYDROLYSIS OF IONS Reactions of water Water takes part in
Water takes part in many kinds of reaction not all of which go to completion CO3 2-+ H2O HCO3 - +OH- (12) Reactions (1) to (6) involve breaking |
|
Solutions to Chem 321 Work Group Set 4 Questions - CSUN
determine whether base hydrolysis or acid dissociation is more important This is done by comparing the equilibrium constants for these two reactions Ka HCO3 |
Does HCO3 hydrolyze in water?
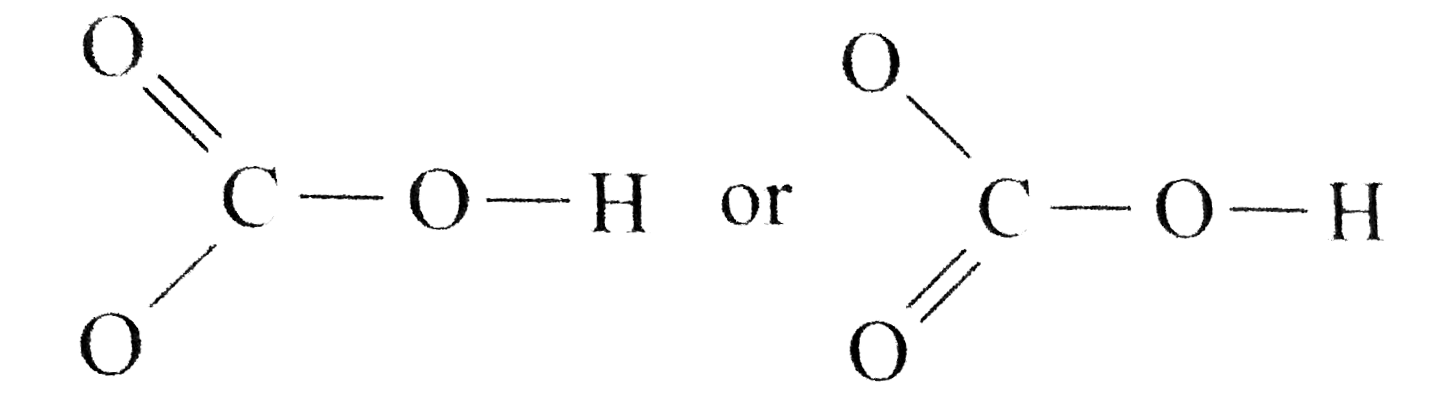
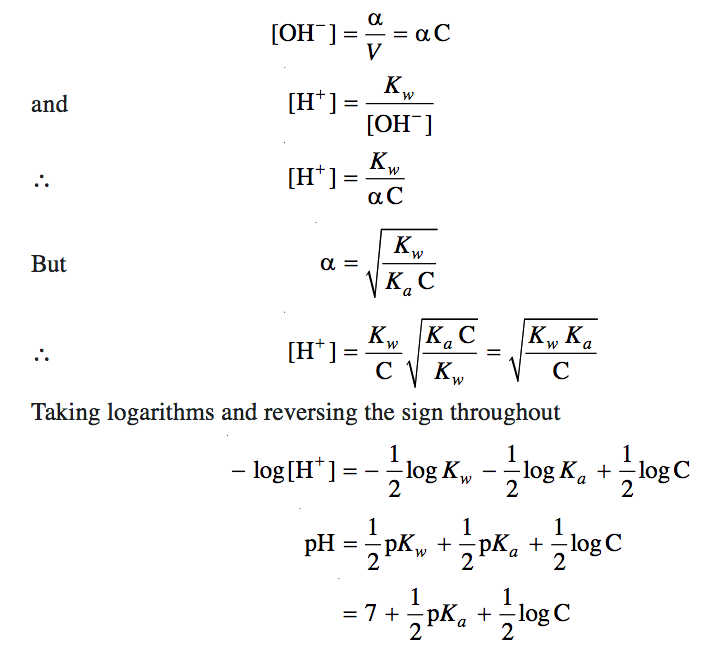
The important thing to remember for both anions and cations within a hydrolysis reaction is to know their origin. The anion, HCO3-, reacts with water to produce carbonic acid and hydroxide ion. Since HCO3- is an anion from a weak acid, the hydroxide ions are available in the water, making it more basic.- Degree of hydrolysis is given as: h=?KhC=?5.5??100.1=(55??10)1/2=7.42??5. Q. Calculate the degree of hydrolysis (h) of a 0.1 M sodium acetate solution at 298 K.
|
Hydrolysis - SSS Chemistry - D Colgur
(Look for ion on the LEFT SIDE of the acid table, read Ka on the right ) -) and write the net-ionic equation for it -) > Ka (HCO3 -) , the ion HCO3 - predominantly undergoes BASE HYDROLYSIS |
|
Reactions of Salt with Water
equilibrium constant of the hydrolysis reaction Setting the Will the solution of such a salt be acidic due to the reaction: HCO3 - + H2O CO3 2- + H3O + |
|
Hydrolysis: - 911 Metallurgist
-) > Ka (HCO3 -) , the ion HCO3 - predominantly undergoes BASE HYDROLYSIS ( 2 3 x 10-8 ) (5 6 x 10-11 ) And the net-ionic equation for the predominant |
|
Hydrolysis - Arcuric Acid
spectators are eliminated in net ionic equations for hydrolysis Process – if given salt And the net-ionic equation for the predominant hydrolysis is: HCO3 |
|
NaHCO3 + H2O Na+ + HCO3 - Cal State LA
1 Equilibrium constant expressions (Keq, Ka, Kb, Ksp, Kf, Kd, etc ) 2 Mass- balance equations |
|
Chapter 16 Acid-Base Equilibria • Acids and bases - publicasuedu
CO3 2-(aq) + H2O(l) r OH-(aq) + HCO3 -(aq) Acid-Base Neutralization Write a balanced equation showing how the following substances behave as acids in Hydrolysis is not observed with ions derived from strong acids or bases: |
|
Acids and Bases (WR) - Centennial Christian School
Write a hydrolysis equation and explain why this salt causes the indicator to 7) The two reactants in an acid-base reaction are HNO2(aq) and HCO3 - (aq) |
|
Sample Exercise 161 Identifying Conjugate Acids and Bases
The two bases in the equation are CO3 2–, the base conjugate base of the weak acid CH3COOH and will hydrolyze to produce OH– ions, thereby making the |





















![PDF] Infrared spectroscopy of hydrated bicarbonate anion clusters PDF] Infrared spectroscopy of hydrated bicarbonate anion clusters](https://ars.els-cdn.com/content/image/1-s2.0-S0925857420301737-gr2.jpg)




