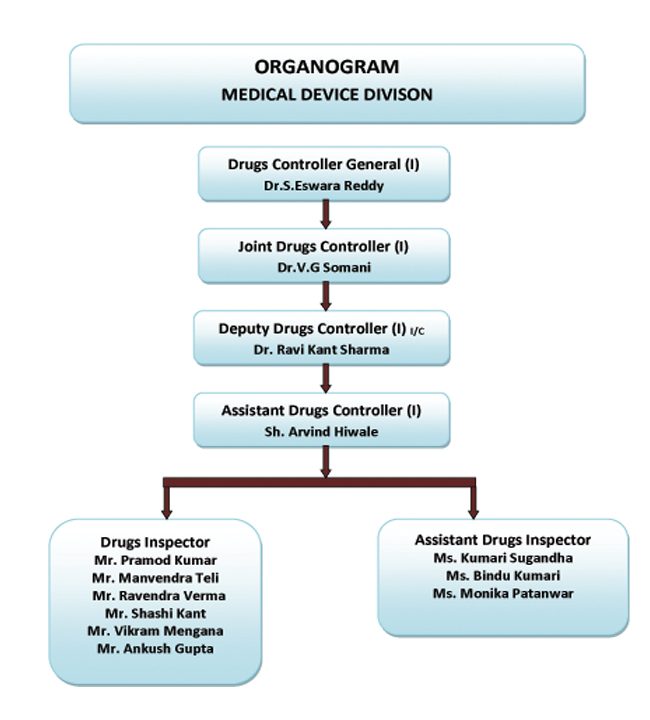
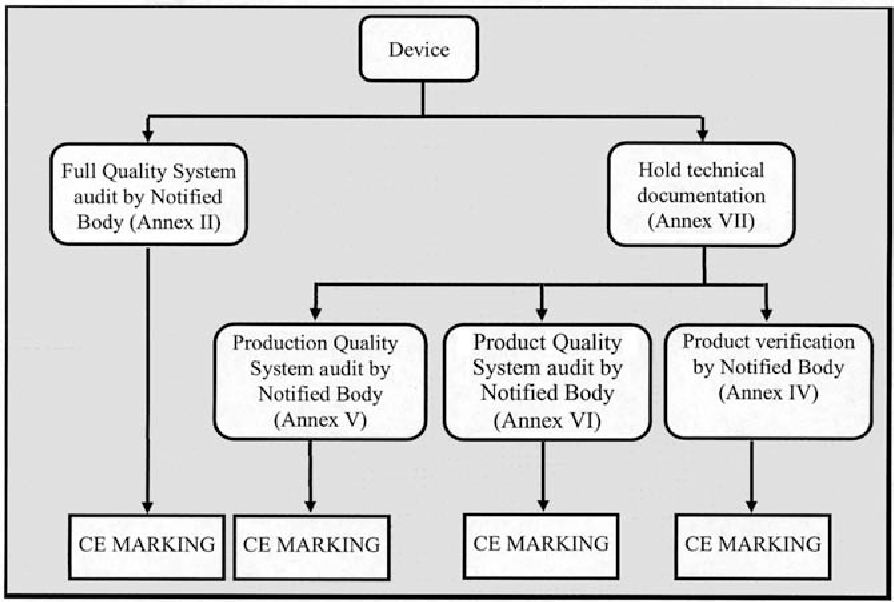
health products (medical devices) regulations
|
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010
These Regulations may be cited as the Health Products (Medical Devices) Regulations 2010 and shall come into operation on 10th August 2010. Definitions. 2. In |
|
Policy for Device Software Functions and Mobile Medical
U.S. Department of Health and Human Services. Food and Drug Administration. Center for Devices and Radiological Health. Center for Biologics Evaluation and |
|
An Introduction to FDAs Regulation of Medical Devices
Center for Devices and Radiological Health FDA Device Regulatory Authority: Laws. • 1976: Medical Device Amendments to Federal Food Drug |
|
Guide for Medical Device Manufacturers on Auditing by the Health
23-Aug-2010 The Health Products Regulatory Authority (HPRA) previously the Irish Medicines Board |
|
REGULATION (EU) 2017/ 745 OF THE EUROPEAN PARLIAMENT
05-May-2017 medical devices which ensures a high level of safety and health whilst ... group of products falls within the scope of this Regulation. |
|
GN-08: Guidance on Medical Device Advertisements and Sales
The relevant legislative control for the advertisement of medical devices is included in the following legislation: Health Products Act. - Part V Advertisement |
|
The EUSFTA and Beyond: Trade in Pharmaceutical Products and
Health Products. (Medical Devices). Regulations 2010. » Generally only licensed medical device dealers are allowed to manufacture |
|
Preparing for the future: The new European Union medical devices
Since the 1990s regulation of the medical device industry in Europe has been as per the regulations for health and safety on pharmaceutical products |
|
Singapore Medical Devices Regulatory Updates
Health Products (Medical Devices) Regulations 2010. • Hierarchy of regulatory requirements. ? Guidance Documents ( Requirements available on the web. |
|
Health Products and Food Branch Inspectorate Guidance Document
01-Jun-2010 Regulations. This includes Human Drugs; Natural Health Products; Medical Devices; Veterinary Drugs;. Blood and Blood Components for ... |
|
MEDICAL DEVICE REGULATIONS
World Health Organization Medical device regulations : global overview and guiding principles 1 Equipment and supplies – legislation 2 Equipment and |
|
Health Products (Medical Devices) Regulations 2010 - Singapore
These Regulations may be cited as the Health Products (Medical Devices) Regulations 2010 and shall come into operation on 10th August 2010 |
|
MDCG 2021-24 Guidance on classification of medical devices
Sections 2 2-2 6 give an overview of some requirements that depend on the class of the device For detailed and exhaustive provisions on each topic refer to |
|
MDCG 2022 – 5 - European Union
MDCG 2022 – 5 Guidance on borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices April 2022 |
|
Medical Devices Regulation - PAHO/WHO
This seminar was designed to introduce Latin American and Caribbean health officials to Canadian U S and European experiences with the regulation of medical |
|
Medical Devices Regulations ( SOR /98-282) - Lawsjusticegcca
PDFFull Document: Medical Devices Regulations [765 KB] Regulations are current to 2023-04-20 and last amended on 2023-02-22 Previous Versions Enabling Act |
|
Medical Devices Regulations ( SOR /98-282) - Lawsjusticegcca
27 avr 2023 · Safety and Effectiveness Requirements 10 A medical device shall be designed and manufactured to be safe and to this end the manufacturer |
|
Medical Devices - Baker McKenzie
The Regulations revoke the previous Health Products (Medical Devices) Regulations 2007 It retains the duties and obligations applicable to manufacturers |
|
An Introduction to FDAs Regulation of Medical Devices
Explain FDA's role in regulating medical devices • Define a medical device and review basics about device classification • Describe five steps to get a |
|
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010
These Regulations may be cited as the Health Products (Medical Devices) Regulations 2010 and shall come into operation on 10th August 2010 Definitions |
What is the ISO for medical device regulations?
Safety and quality are non-negotiable in the medical devices industry, that's why we developed ISO 13485. Regulatory requirements are increasingly stringent throughout every step of a product's life cycle, including service and delivery.What is the MDR Rule 14?
Distributors shall, upon request by a competent authority, provide it with all the information and documentation that is at their disposal and is necessary to demonstrate the conformity of a device.What is the MDR regulation for medical devices?
The Medical Device Reporting (MDR) regulation (21 CFR Part 803) contains mandatory requirements for manufacturers, importers, and device user facilities to report certain device-related adverse events and product problems to the FDA.- The Turkish MDR requires that information accompanying medical devices must be provided in Turkish. Similar requirements also exist in the Member States of the EU.
|
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010 In exercise of the powers conferred by sections 45, 71 and 72 of the Health Products Act, the |
|
MEDICAL DEVICE REGULATIONS - WHO World Health Organization
Equipment and supplies – standards 3 Policy making 4 Risk management 5 Quality control I Title ISBN 92 4 154618 2 (NLM Classification: WA 26) |
|
Singapore - WHO World Health Organization
In vitro diagnostic medical device (IVD) defined: Yes Text: Defined Separately Health Products Act (Medical Devices) Regulations, Third Schedule, Part II, 24 |
|
Medical Devices - Health Sciences Authority
The registrant is the holder of the certificate of registration of the medical device, on behalf of the product owner Import for Personal Use 1 Limit on quantity |
|
MEDICAL DEVICES - Health Sciences Authority
These Regulations are the Health Products (Medical Devices) (Amendment) Regulations 2018 and come into operation on [1 June] 2018 Amendment of |
|
Medical Devices - Baker McKenzie
Healthcare Singapore Medical Devices In August 2010, Singapore implemented the Health Products (Medical Devices) Regulations 2010, pursuant to the |
|
COVID-19 Medical Devices: Singapore - Bird & Bird
barriers and exempting products from regulatory approval requirements Products Act and the Health Products (Medical Devices) Regulation 2010, including |
|
Global Regulatory Requirements for Medical Devices - DiVA
Medical devices are becoming more important in the health care sector One of the major 1 4 Regulation of Medical Devices 4 1 4 Product Registration |