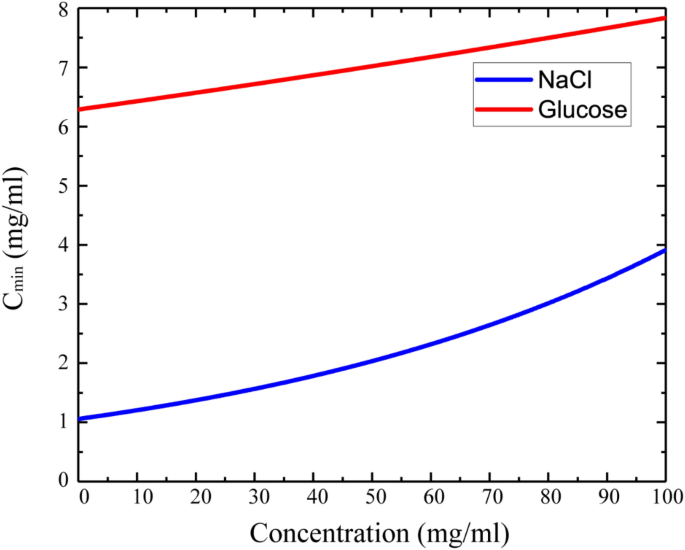
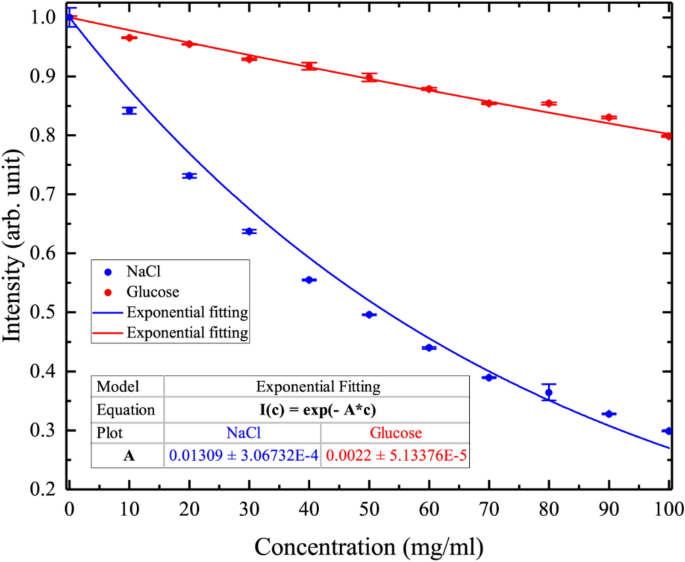
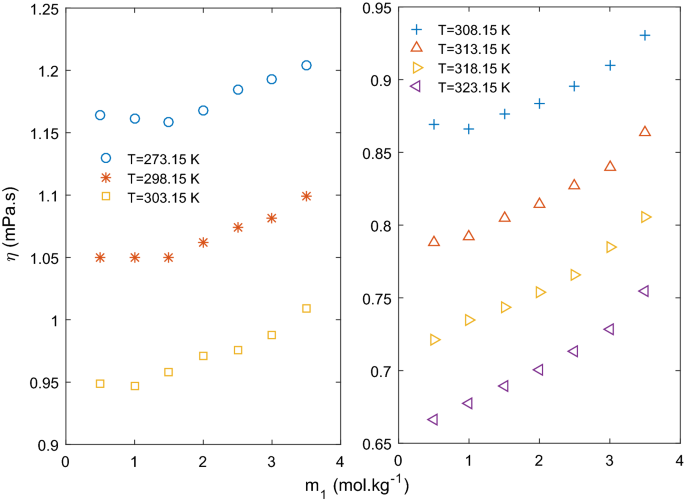
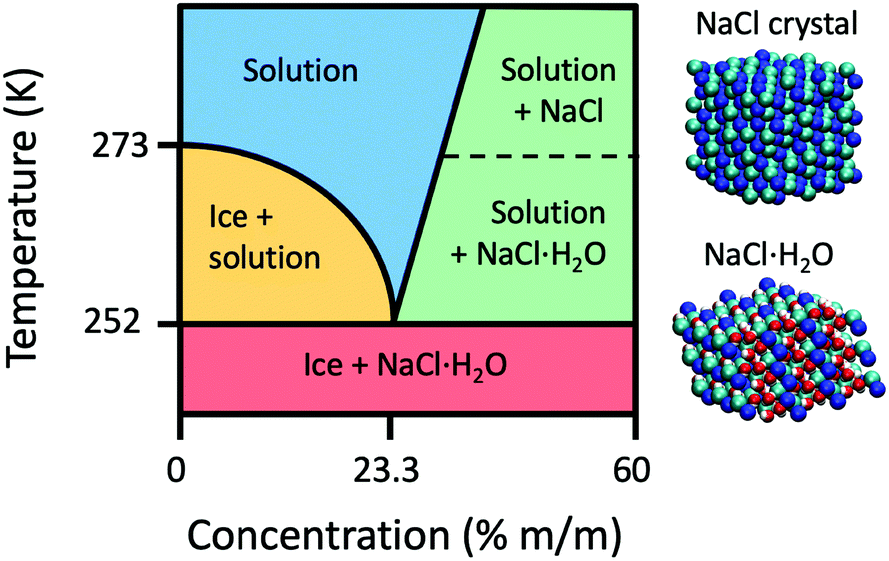
how do 0.5m and 2.0m aqueous solutions of nacl differ
|
Hydrophobically associating polyacrylamides and their partially
In dilute solution large differences molecular non-covalent hydrophobic junctions would ... H00C12-1.0 samples directly in 0.5M NaCl aqueous solution ... |
|
Laboratory Solution Preparation
volume (V2) and molarity (M2) the final solution should be. 142.02. 0.5 M. 71.0 g. Sodium sulfide. 2.0 M. 48.0 g§. Na2S • 9H2O. |
|
Long-Cycling Aqueous Organic Redox Flow Battery (AORFB
14 déc. 2016 energy storage solutions.1?3 Redox flow batteries (RFBs) have ... 0.5 M for FcNCl in 2.0 M NaCl electrolyte (corresponding to. |
|
A new view on gold speciation in sulfur-bearing hydrothermal fluids
6 juil. 2019 aqueous solution at elevated temperatures and all available ... sulfide concentration ranges (0.05-2.0 m H2S/HS-) of this study |
|
Chapter Four: Reactions in Aqueous Solution
Electrolyte: Substance whose aqueous solutions contain ions (e.g. NaCl). • Nonelectrolyte: Substance that does not form ions in solution (e.g.. C12H22O11) |
|
A new view on gold speciation in sulfur-bearing hydrothermal fluids
aqueous solution at elevated temperatures and all available information on their sulfide concentration ranges (0.05-2.0 m H2S/HS-) of this study |
|
Fractionation of Deoxyribonucleic Acids on Columns of Anion
5 août 2021 changers differ in basic strength (pKa) in 0.5 M. NaCl DEAE = 9.5; ECTEOLA = ... 0.5 to 2.0 M NaCl to above 8 before additional DNA was. |
|
Etude expérimentale du fractionnement isotopique du fer aux
2 mars 2011 between hematite and aqueous solution at hydrothermal conditions” ... multiplient et diffèrent les unes des autres selon les auteurs et les ... |
|
Article - Designer Two-Electron Storage Viologen Anolyte Materials
Aqueous organic redox flow batteries (AORFBs) are highly attractive for large- 2.0 M in 2.0 M NaCl solution) redox stability |
|
B75 Lab manual_ss_pt1
In this activity you will learn how to calculate and make copper sulfate (CuSO4) solutions of differing mass/volume concentrations. Solutions are prepared with |
|
Name: Solutions Review
1) Both the freezing point and boiling point of the solution are higher 2) Both the freezing point and boiling point of the solution are lower |
|
Solutions - Rodens AP Chemistry
(a) Describe the change in entropy when sodium chloride dissociates into aqueous particles (b) Two saturated aqueous NaCl solutions one at20"C and one at 50"C |
|
Sans titre
WebConsider the two solutions:I 0 5 M NaCl aqueous solution at 25^oC ; NaCl is completely ionized II 2 0 M C6H5COOH in benzene at 25^oC;C6H5COOH dimerizes |
|
Concentration of Solutions and Molarity - Denton ISD
A saline solution contains 0 90 g of NaCl per 100 mL of solution What is its molarity? 1) convert grams to moles 0 90 g NaCl / 58 5 g/mol = 0 015 |
|
Key
0 5M = 1 0M C 1 0 mol per 8 0 L = 0 125 b 4 0 mol per 4 0 L C |
|
Toxins Section 3 Practice Test (Lessons 13
Draw molecular views for 1 0 M NaCl 2 0 M NaCl and 1 0 M Na?S Use different symbols for each type of ion Circle the solution(s) with the least total number |
|
(2) Preparation and Dilution of Solutions
A simple solution is basically two substances that are evenly mixed together ? One of them is called the soluteand the other is the solvent |
|
Chemistry The Central Science 11th edition
Solutions are defined as homogeneous mixtures of two or more substances • Solvent: present in the greater quantities and is used to dissolve the solute |
|
Solutions
Aqueous solutions are mostly used in the laboratories Water is called a universal solvent because it dissolves majority of compounds present in earth's crust |
|
2 Amount and concentration: making and diluting solutions
The concentration of this solution could be described as 0 009 g/mL or 9 mg/mL Alternatively you could notice that if 0 9g is dissolved in 100mL |
How do 0.5 mm and 2.0 M aqueous solutions of NaCl differ?
How do 0.5M and 2.0M aqueous solutions of NaCl differ? The 2.0M solution contains more moles of NaCl per volume of water than the 0.5M solution. Under what conditions might a chemist describe a solution in terms of molality?How many moles of NaCl are in 2.0 L of a 0.5 M solution?
If you know the Molecular weight of NaCl you multiply with 0.5 to get the answer. Required number of moles =2?0.25=0.5 moles.How do you make a 0.5 M solution of NaCl?
For example, if you wanted a 0.5 M solution, you would use 0.5 x 58.44 g/mol of NaCl in 1 L of solution or 29.22 g of NaCl.- Reason for 1m more concentrated: Because 1 molar solution contains 1 mole of solute in 1 litre of the solution, which includes both solute and solvent, it is more concentrated than 1 molar solution. Consequently, less than 1000 grams of water serve as the solvent mass.
|
14 Mixtures and Solutions
2 Distinguish between suspensions and colloids Suspension particles are larger than colloidal mol HCl 5M What is the mole fraction of NaOH in an aqueous increasing temperature? 60 0 100 20 Solubility (g/100 g H 2 O) 10 30 20 |
|
Solutions - NCERT
describe the formation of different Solutions are homogeneous mixtures of two or more than two 2015-16(20/01/2015) 1 mol (74 5 g) of KCl is dissolved in 1 kg of water 0 represents the vapour pressure of the pure component 2 |
|
Laboratory Math II: Solutions and Dilutions - NIH Office of Intramural
While you may already make solutions in the lab by following recipes, Concentration can be recorded and reported in many different ways depending on the Add that to a container and bring the volume to five liters with water This means that you add 10 milliliters of the 5M NaCl stock solution to a container and |
|
H Hw and SG keypdf
water • All beakers are kept at 20 °C • All solutions are stirred for 2 hours Beakers A-E in Model 1 are depicted as representing five different or separate solutions A 0 5 M KCl solution contains 74 55 g of KCl (molar mass 74 55 g/ mol) in |
|
Electrolyte Solutions: Thermodynamics, Crystallization - CORE
18 déc 2017 · influence of salts on the vapor pressure of aqueous solutions of organic A large number of different concentration units are used to present molality units give practical numbers, often between 0 and 20 for most salts, while the 0 0 02 0 04 0 06 0 08 0 1 NaCl mole fraction KC l m o le a c tio n a b |
|
CO2 solubility in water and brines - CO2 - CATO
17 sept 2009 · Deliverable WP 4 1-3-05 3 2 CO2 solubility modelled properties of carbon dioxide, water and carbon dioxide-water ± NaCl mixtures over a wide range of Though there is little difference in the quality of the various EOS mentioned aqueous solutions from 0 to 260°C and from 0 to 2000 bar total |