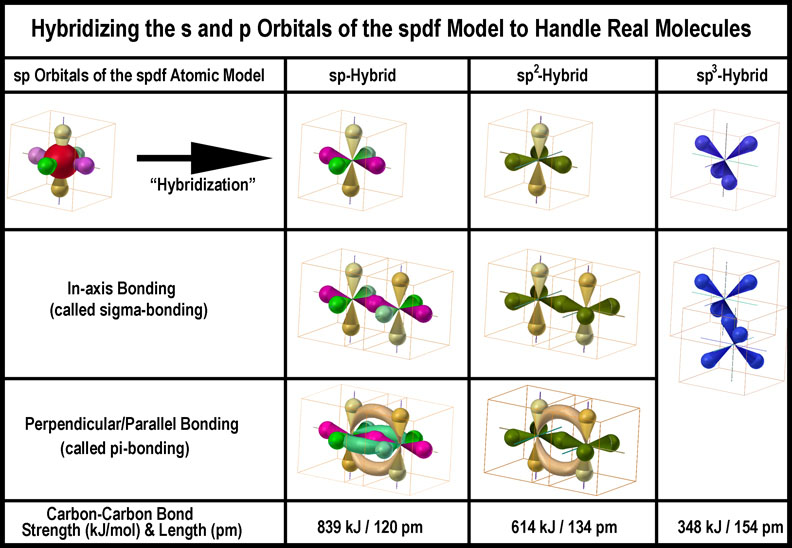
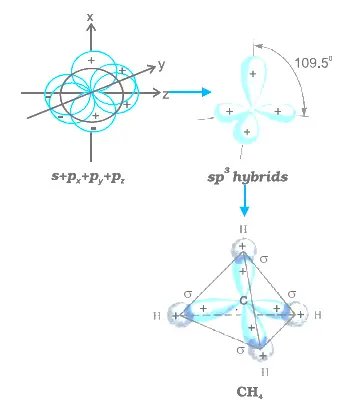
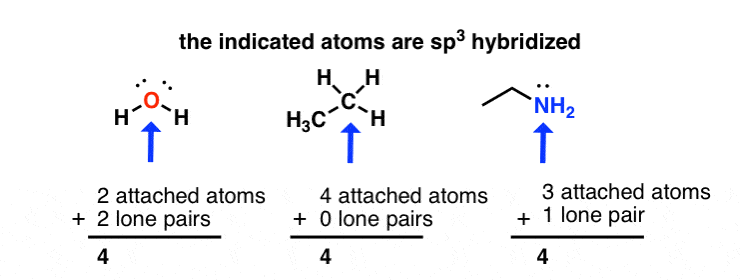
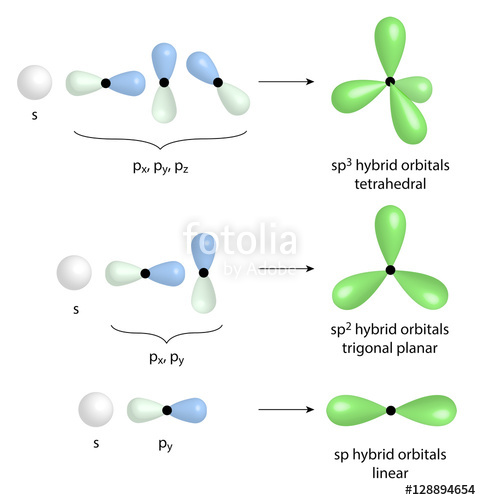
hybridization of atomic orbitals
|
Hybridization of atomic orbitals In general VSEPR predicts the
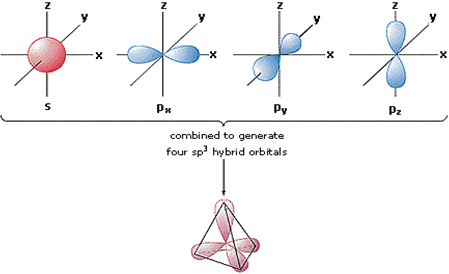
Hybridization of atomic orbitals Imagine an orbital (containing 1 electron) from one ... the carbon atom to overlap with the four orbitals of. |
|
Hybridization of Atomic Orbitals
As examples each of the C |
|
Chemical Bonding II: Molecular Geometry and Hybridization of
pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals. |
|
When atoms bond to form molecules they use molecular orbitals
Carbon has 4 valence electrons. Add these electrons to the atomic and molecular orbitals. This hybridization gives tetrahedral geometry. With this hybridization |
|
CHAPTER 14 COVALENT BONDING: ORBITALS
The carbon atoms are sp3 hybridized. The six C?H sigma bonds are formed from overlap of the sp3 hybrid orbitals on C with the 1s atomic orbitals from the |
|
Retire the Hybrid Atomic Orbital? Not So Fast
12 mars 2012 two or more atoms interact. Although hybridization of atomic orbitals is not necessary for valence bond theory in general (for. |
|
Chemical Bonding II: Molecular Geometry and Hybridization of
Hybridization of Atomic Orbitals. Chapter 10. Linear. 180o. Trigonal planar. 120o. Tetrahedral. 109.5o. Trigonal. Bipyramidal. 120 and 90o. Octahedral. |
|
Chemical Bonding II: Molecular Geometry and Hybridization of
Hybridization of Atomic Orbitals (10.4). Hybridization in Molecules Containing. Double and Triple Bonds (10.5). Molecular Orbital Theory (10.6) |
|
Chemical Bonding II: Molecular Geometry and Hybridization of
4 nov. 2010 Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. |
|
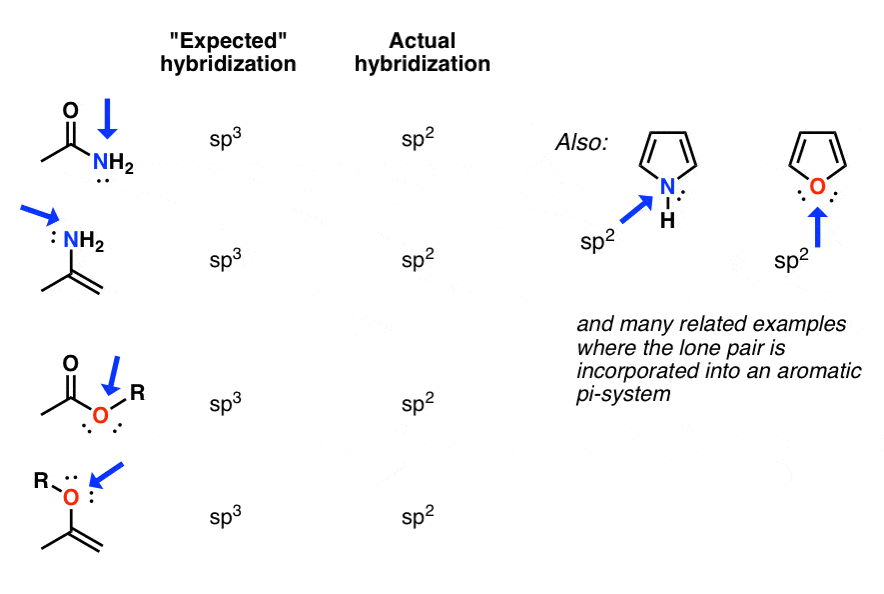
Hybrid Atomic Orbitals in Organic Chemistry. Part 2: Critique of
Hybridization schemes require a prior knowledge of the general molecular geometry before assigning the type of orbitals. Moreover each scheme uses a disparate |
|
1 Hybridization and atomic orbitals - MIT OpenCourseWare
Hybridization and atomic orbitals Lewis dot diagrams and VSEPR are powerful tools to think about electronic con?guration and molecular shape but neither tool o?ers a way to predict bond stability The strength of a covalent bond is proportional to the amount of overlap between electronic orbitals Consider BeH 2 |
|
Hybrid Molecular Orbitals - University of Illinois Urbana
Hybrid orbitals are constructed from valence atomic orbitals and used to make sigma bonds between atoms Sigma bonds are formed by the overlap of orbitals that are pointing directly towards one another If two atoms are connected by a sigma bond rotating one of the atoms around the bond axis doesn't break the bond |
|
Worksheet 14 - Hybridization molecular orbitals atomic
Worksheet 14 - Hybridization When atoms bond to form molecules they use molecular orbitals These are formed through the hybridization of the atomic orbitals that we have already discussed s p and d orbitals The hybridized molecular orbitals have different shapes and energy levels than the atomic orbitals The number of molecular orbitals |
|
Searches related to hybridization of atomic orbitals filetype:pdf
Hybrid atomic orbitals Hybridization History Orbitals A B S T R A C T Hybrid atomic orbitals have become a central component of organic chemistry The history of these hybrids and hybridization as a process is discussed; this history is divided into a timeline from before 1931 to the present day The |
What are the types of hybridization of orbitals?
- Although there are several types of Orbital Hybridization, the following three are the most simple and main types. The Hybridization in which one S and three P orbitals intermix and form four hybrid orbitals of the same shape and energy is known as SP 3 Hybridization. Each hybrid orbital is known as an SP 3 hybrid orbital.
How does hybridization allow atoms to achieve the octet rule?
- This would create a Lewis structure with a single electron on each of the four sides of carbon, allowing it to form four covalent bonds. This means that carbon atoms will be able to achieve the octet rule when they form four bonds. What are some examples of orbital hybridization?
What are the types of hybridization?
- The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. Linear: Two electron groups are involved resulting in sp hybridization, the angle between the orbitals is 180°.
What is the process of hybridization?
- Hybridization Hybridization, as related to genomics, is the process in which two complementary single-stranded DNA and/or RNA molecules bond together to form a double-stranded molecule. The bonding is dependent on the appropriate base-pairing across the two single-stranded molecules.
|
Hybridization of Atomic Orbitals - Organic Chemistry basics
Hybridization of Atomic Orbitals The atomic structures, from the Periodic Table, of atoms such orbitals (sp3) that will make sigma bonds with H atoms in H 2 |
|
Hybridization of atomic orbitals In general VSEPR predicts the
According to this view four orbitals are needed from the carbon atom to overlap with the four orbitals of the hydrogen atoms The ground state of C: 1s2 2s2 2p2 |
|
CHAPTER 14 COVALENT BONDING: ORBITALS
The carbon atoms are sp3 hybridized The six C‒H sigma bonds are formed from overlap of the sp3 hybrid orbitals on C with the 1s atomic orbitals from the |
|
Hybrid orbitals
11 1 Valence Bond (VB) Theory and Orbital Hybridization 11 2 Modes of Orbital Overlap and the Types of Covalent Bonds 11 3 Molecular Orbital (MO) Theory |
|
Molecular Geometry and Hybridization of Atomic Orbitals - CDN
Valence Bond Theory (10 3) Hybridization of Atomic Orbitals (10 4) Hybridization in Molecules Containing Double and Triple Bonds (10 5) Molecular Orbital |
|
Chapter II Hybridization in hydrocarbons
❖ Number of combined atomic orbitals = number of resulted hybrid orbitals Page 4 1- sp3 Hybridization: combination of an s orbital with 3 p orbitals |
|
Molecular Geometry and Hybridization of Atomic Orbitals
Hybrid orbitals get 1 electron for a σ-bond, 2 electrons for a lone pair sp3 hybridization for H 2 O Needed to form 2 sigma bonds |