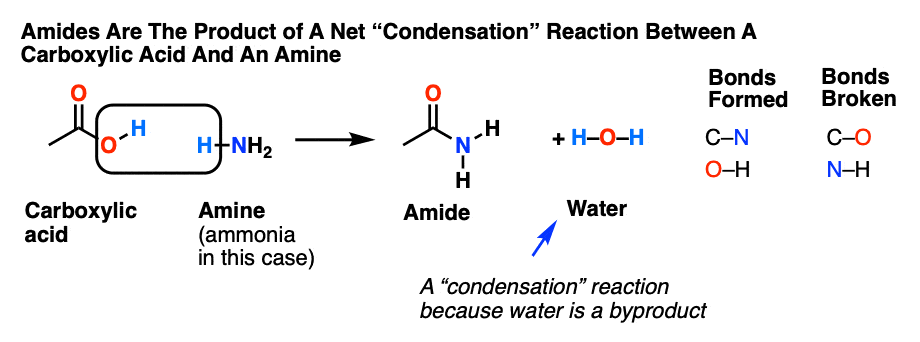
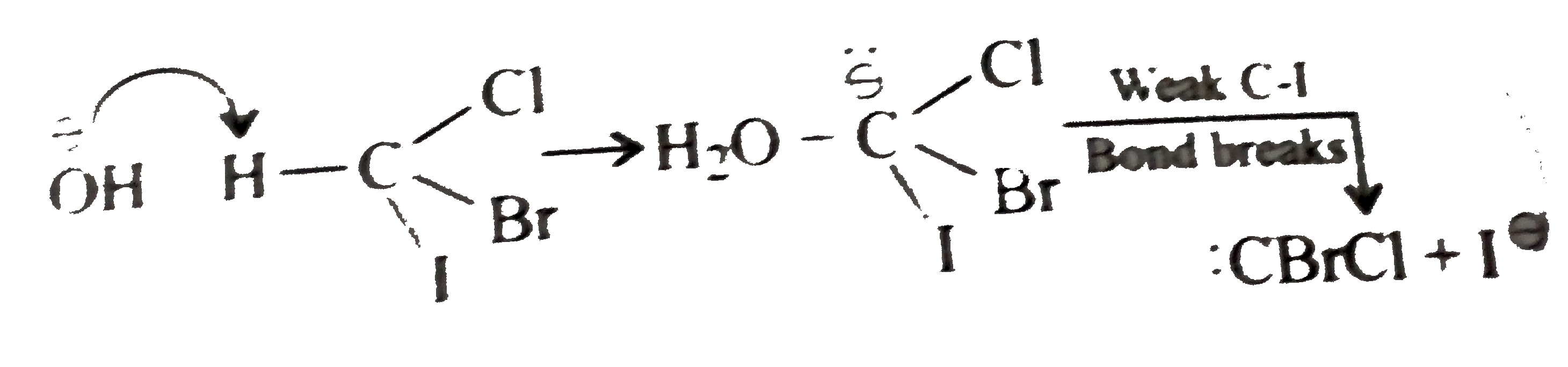hydrolysis of benzamide in acidic and basic medium
|
HYDROLYSIS REACTIONS
19 Şub 2018 (Hydrolysis is carried out using an acid or base catalyser in aqueous medium.) The task of the catalysers is to facilitate electron transfer by ... |
|
Stereo-electronic Effects in the Alkaline Hydrolysis of Benzamides
Found: C 72.93; H |
|
A mild alkaline hydrolysis of N- and NN-substituted amides and
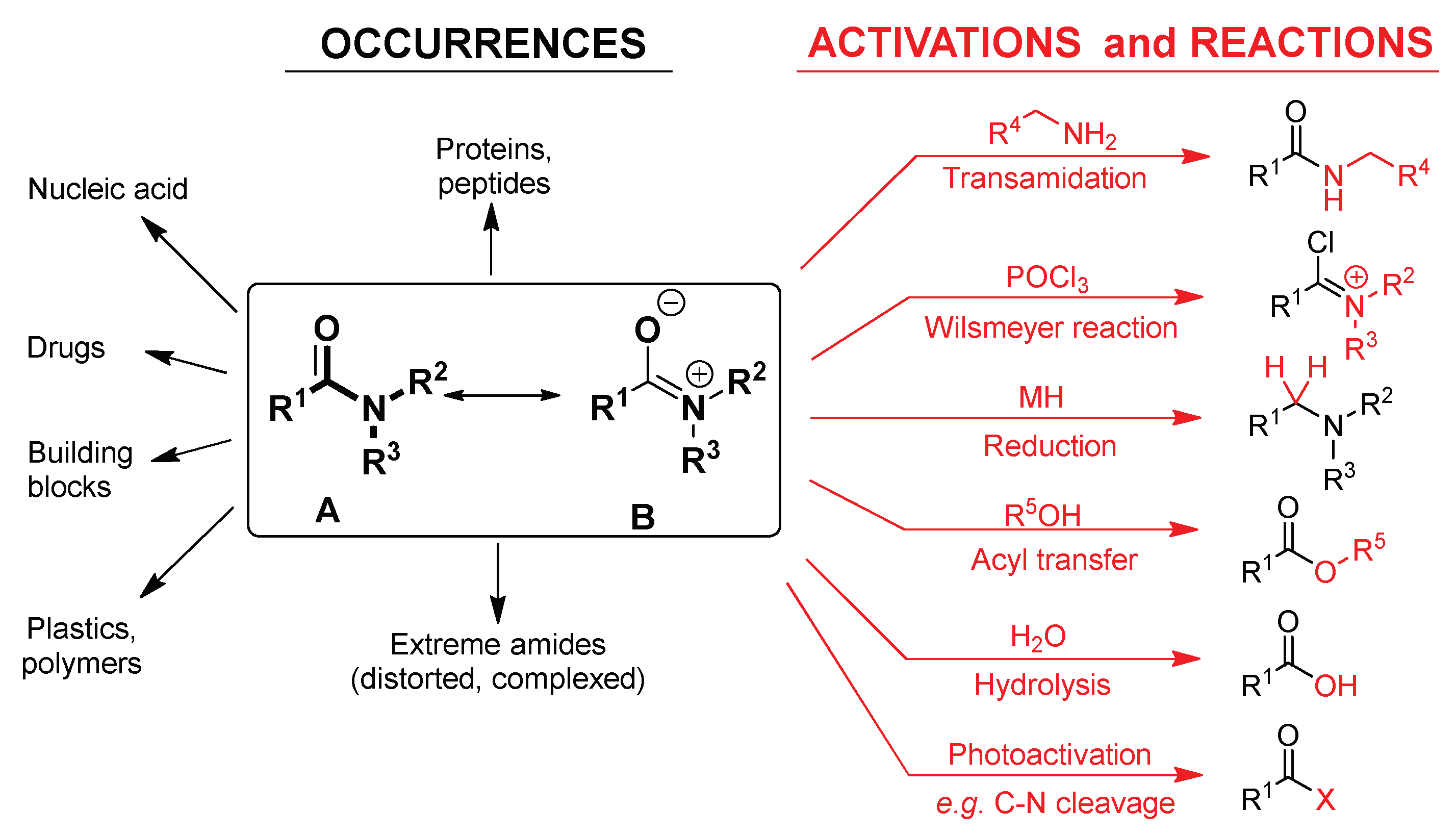
In general nitriles and amides are exceptionally stable to acid and basic hydrolysis and classically they are hydrolyzed under vigorous reaction conditions |
|
Structure medium
https://pubs.acs.org/doi/pdf/10.1021/ja00753a042 |
|
Activation Energies of the Hydrolysis of Esters and Amides Involving
acidic and basic hydrolysis of ethyl benzoate underidentical conditions of hydrolysis in the alkaline hydrolysis of benzamide in water: A 40.7°; B |
|
Structure medium
http://pubs.acs.org/doi/pdf/10.1021/ja00753a042 |
|
Standard Input
45 °C in alkaline solution at ionic strength 1. The pH-rate profiles show the alkaline hydrolysis of aliphatic amides. Most of this work is summarized ... |
|
Transition state activity coefficients in the acid-catalyzed hydrolysis
amide hydrolysis in acidic solutions (cf. ref. 14). The only exception is N hydrolysis as a function of medium composition is only compatible with the ... |
|
Acylium Ion Formation in the Reactions of Carboxylic Acid
anism of the acid- and base-catalyzed hydrolysis of esters and amides of benzoic acid.2-5. It was thought that the kinetic and oxygen exchange criteria |
|
THE ALKALINE HYDROLYSIS OF N-ACYLTHIOUREAS
1 Mar 1974 medium: in weakly alkaline solution (pH 9.02) the Hammett reaction ... hydrolysis of benzamide eq. 7 would yield. So |
|
Stereo-electronic Effects in the Alkaline Hydrolysis of Benzamides
Benzamides and Benzonitriles (Pseudo)-first-order rate constants for the alkaline hydrolysis of ¿-substituted ... vals the boric acid solution was. |
|
Acid-catalyzed and alkaline hydrolyses of phosphinamides. Lability
reactions.2 In general carboxylic amides are resistant to hydrolysis; high temperatures and strongly acidic or basic conditions are required. |
|
Activation Energies of the Hydrolysis of Esters and Amides Involving
acidic and basic hydrolysis of ethyl benzoate underidentical conditions of temperature and solvent show ratios of kh/ke that differ by a factor ofabout two |
|
Acylium Ion Formation in the Reactions of Carboxylic Acid
The kinetics and oxygenexchange in the diazotization of benzamide were investigated. anism of the acid- and base-catalyzed hydrolysis of. |
|
Standard Input
acetamide have been hydrolyzed at 25 °C or. 45 °C in alkaline solution at ionic strength 1. The pH-rate profiles show that the rate at high pH values is |
|
Intramolecular Catalysis of Hydrolytic Reactions. III. Intramolecular
Hydrolysis of Benzamide and. Phthalamic Acid. ---ki sec.-1----. Amide. 0.001 M HC1 product and the solvent is too fast in this medium. |
|
Transition state activity coefficients in the acid-catalyzed hydrolysis
hydrolysis of benzamide and its N-alkyl derivatives. medium dependence of log f $+ is observed. ... Williams (2) on the acidic hydrolysis of NN-. |
|
Room temperature hydrolysis of benzamidines and - ChemRxiv
Benzamidinium containing molecules hydrolyze relatively rapidly at room temperature in weakly basic water but do not appear to oxidize under these conditions The rate of hydrolysis increases with increasing pH primarily due to the effect of pH on the protonation state of the benzamidinium |
|
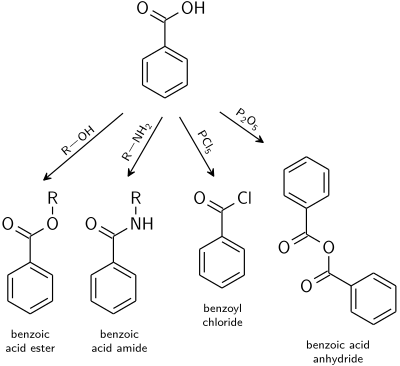
Synthesis and stability of strongly acidic benzamide derivatives
benzamide 9d Hence in the acid-catalyzed hydrolysis reaction of 12 the 4-bromo-N-triflylbenzamide (9d) was only detected as a trace as 9d converted faster to 4-bromobenzoic acid than com-pound 12 converted to 9d In contrast 0 5 M aqueous NaOH rapidly hydrolyzed the NN’-bis(triflyl)benzimidamide 12 to yield the base-stable N |
|
Searches related to hydrolysis of benzamide in acidic and basic medium filetype:pdf
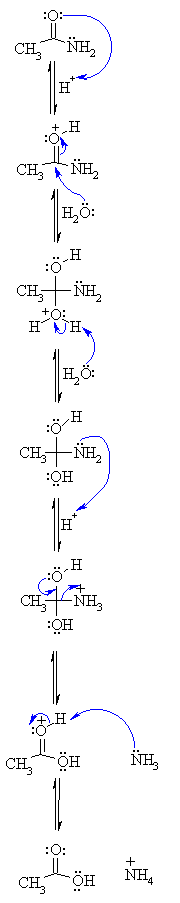
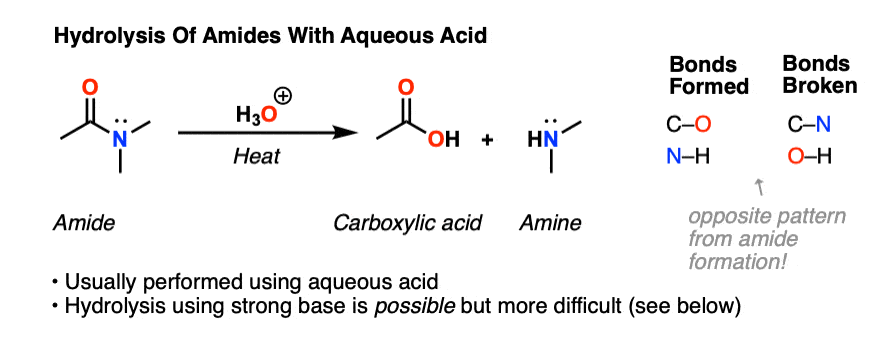
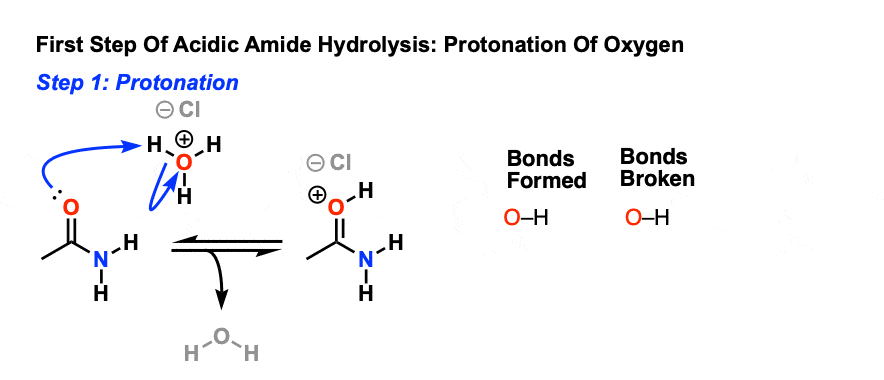
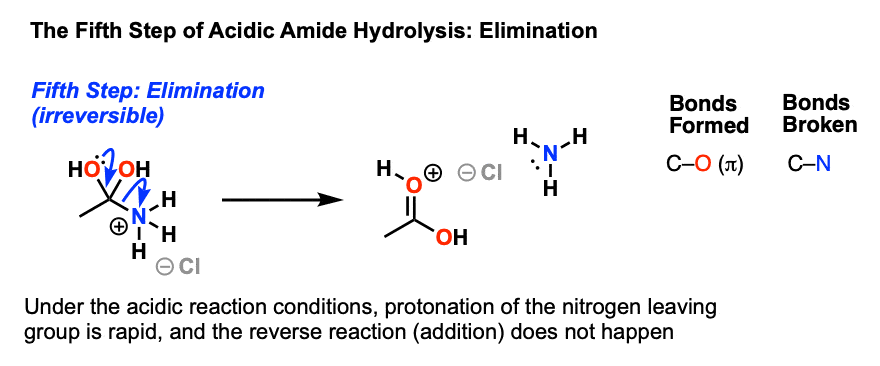
Acid-Mediated Hydrolysis of Amides This reaction mechanism closely resembles the acid-catalysed hydrolysis of methyl esters Proton transfer steps again increase the electrophilicity of the amide carbonyl group and alsogenerate a better leaving group (an amine instead of an amide): |
Does excess acidity affect hydrolysis rate of benzamides and methylbenzimidatium ions?
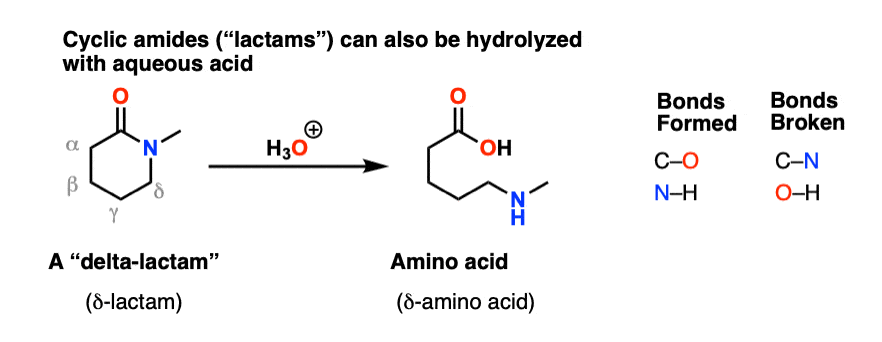
- The excess acidity method has been applied to hydrolysis rate data for a number of benzamides, methylbenzimidatium ions, and lactams, obtained as a function of sulfuric acid concentration and temperature. All of the substrates studied except P-propiolactam
Does benzamide have a bisul- fate mechanism at high acidity?
- Benzamide is the only compound in the study that has been adequately investigated at high acidity. Indications of a bisul- fate mechanism could be seen for 2 and 3 also, but not enough data points at high acidity were present to enable parameters to be calculated.
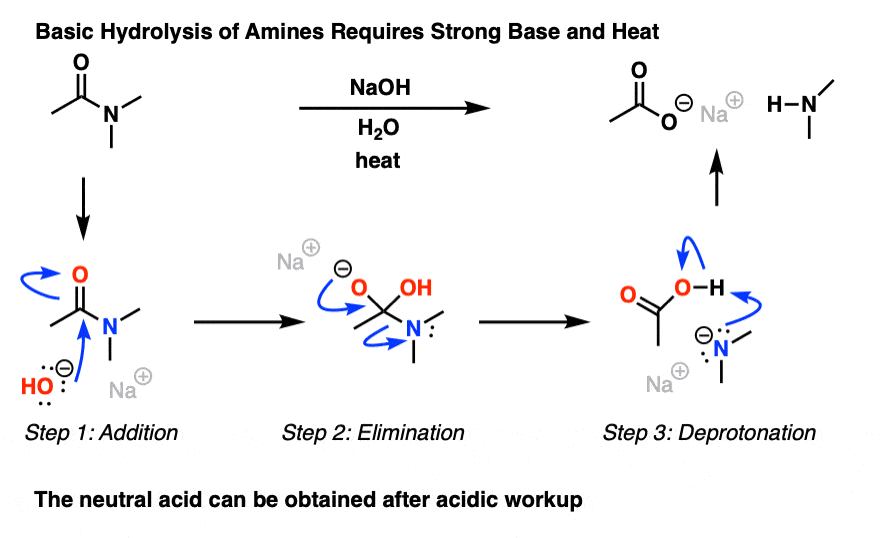
What is the mechanism of alkaline amide hydrolysis?
- The mechanism of the alkaline amide hydrolysis has been intensively investigated. addition of the hydroxide, could regenerate the amide than give the hydrolysis products. exchange is generally faster than hydrolysis (Scheme 1). Scheme 1. Alkaline hydrolysis reaction and oxygen exchange (where R' = H, alkyl, aryl).
How is amide formed if a substrate is a benzimidatium ion?
- If the substrate is a benzimidatium ion, amide formation is the result; McClelland and Potter have shown that the hydrolysis of 0-ethyl-N,N-dimethyl-4- nitrobenzimidatium ion in 48% H2S04at 25OC gives 10% amide product, and that all of it is formed via the P pathway in eq. , as shown by 180 exchange (40).
|
Lecture 6: Hydrolysis Reactions of Esters and Amides
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions; • account for the irreversibility of the hydrolysis reaction under basic |
|
Comparative Stability of Benzamide, Salicylamide, and Some N
In the acid hydrolysis of the amides, effects on the hydrolysis of amides in concentrated acid In basic medium, the absorption peak for these compounds |
|
Synthesis and stability of strongly acidic benzamide - DTU Orbit
27 fév 2018 · Keywords: benzoic acid; cross-coupling; hydrolysis; SNAr; Studies of stability under acidic and basic conditions are also reported 523 |














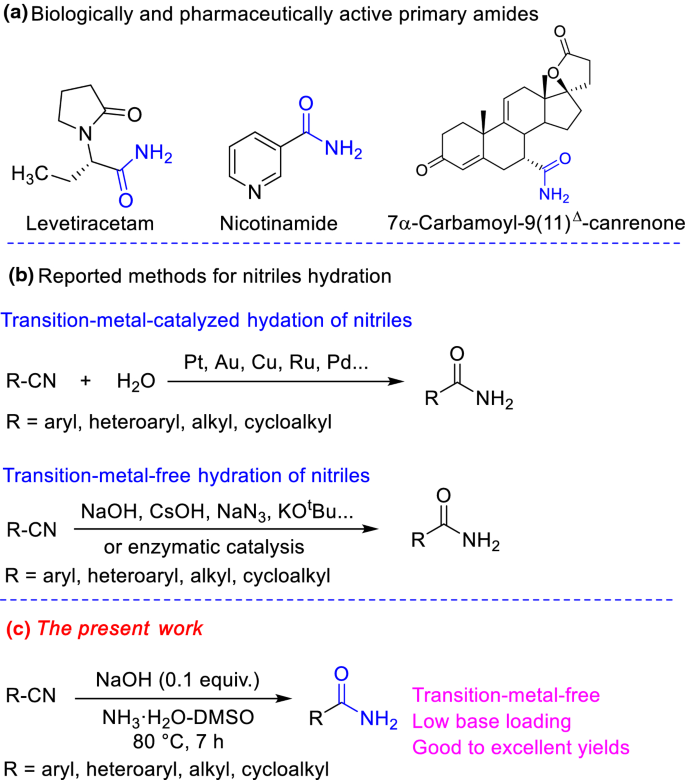




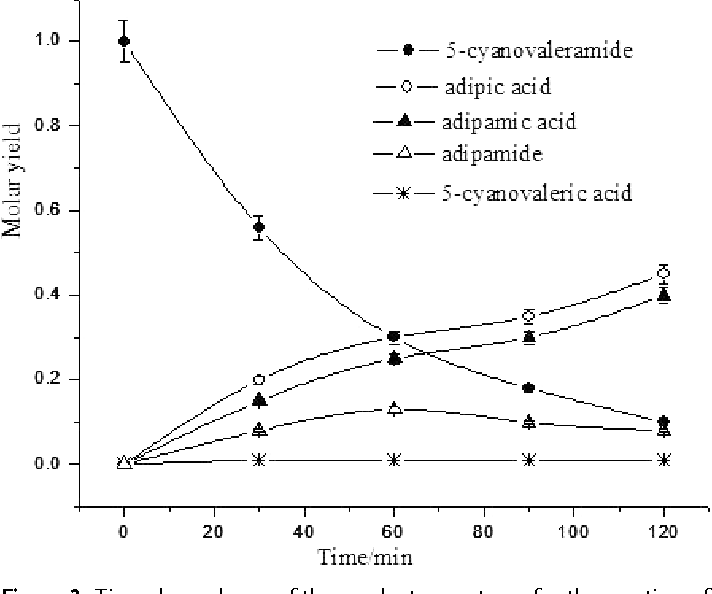
![PDF] Green Medium for the Hydrolysis of 5-Cyanovaleramide PDF] Green Medium for the Hydrolysis of 5-Cyanovaleramide](https://d3i71xaburhd42.cloudfront.net/507e41334610e6568ba93fb289a28ff02f405ea7/4-Figure4-1.png)
![PDF] Green Medium for the Hydrolysis of 5-Cyanovaleramide PDF] Green Medium for the Hydrolysis of 5-Cyanovaleramide](https://ars.els-cdn.com/content/image/1-s2.0-S0223523418309437-fx1.jpg)