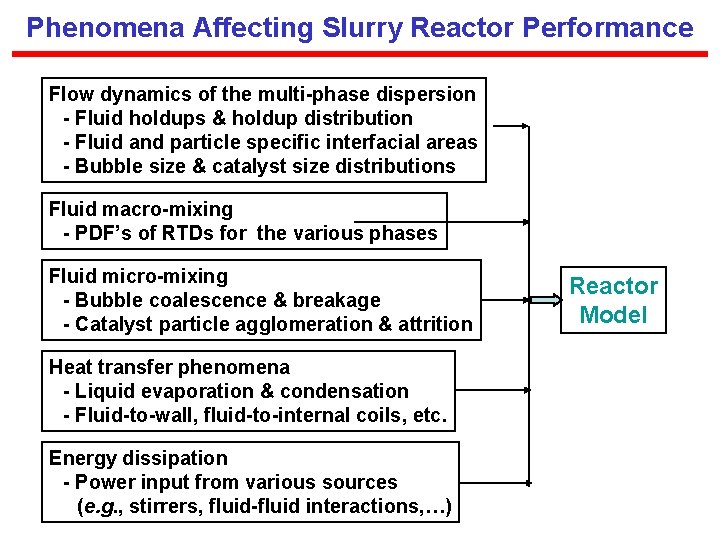
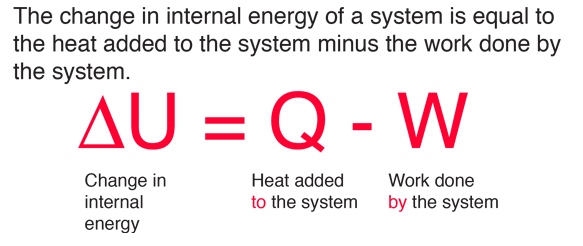
internal energy of mixing
|
Internal wave-driven mixing: governing processes and
9 Oct 2021 are major sources of energy for the internal wave field. 28. • Interactions between internal waves and topography currents |
|
INTERNAL WAVE-DRIVEN MIXING: GOVERNING PROCESSES
Turbulent mixing from breaking oceanic internal waves drives a vertical transport of water are major sources of energy for the internal wave field. |
|
Estimating tidally driven mixing in the deep ocean - LC St. Laurent1
10 Jun 2002 [1] Using a parameterization for internal wave energy flux in a hydrodynamic model for the tides we estimate the global. |
|
Variability of internal tide energy mixing and nitrate fluxes in
11 Dec 2019 Over the continental slopes and in shelf seas the vertical mixing caused by breaking internal tidal waves significantly increases the fluxes of ... |
|
Comparison of different mixing rules for prediction of density and
density and residual internal energy of binary and ternary the modification of the mixing rule for the interaction energy parameter is also necessary ... |
|
Internal waves and vertical mixing in the Storfjorden Polynya Svalbard
24 Dec 2011 Also barotropic tidal energy can be transferred into a baroclinic coastal wave or topo- graphic shelf waves [e.g. Padman et al. |
|
Solutions
internal energy with respect to some reference states as in the case of Note that in equilibrium |
|
Statistical Thermodynamics
Internal Energy and Heat Capacity of the Canonical Ensemble . of mixing which suggests that ln ? is related to entropy. |
|
Helmholtz Energy Transformations of Common Cubic - NIST
The Gibbs energy in the form of the chemical potential is the basis of phase equilibrium calculations in chemical engineering while formulations of the Helmholtz energy have been preferably applied to corelater wide-ranging data and properties of pure fluids with high accracy u Schu formulations are implemented in the current state-of- |
|
Helmholtz Energy Transformations of Common Cubic Equations of Stat
The Gibbs energy of mixing two ideal liquids A and B is: mix G = nRT( x A ln x A + x B ln x B) The corresponding entropy of mixing is: mix S = – nR( x A ln x A + x B ln x B) The corresponding enthalpy of mixing is: mix H = mix G + T mix S = 0 An excess function (XE) is the difference between the observed (real) function of mixing and the ideal |
|
Lecture25 chapt15 Thermodynamics of mixing - Hope College
Thermodynamics of Mixing Let’s think of the lattice model in the book – simulates a liquid of two different things There are N total sites each filled with either an A or a B molecule (sketch lattice) N = NA + NB Let’s think about the entropy of the situation What are the number of arrangements? W = N! / NA!NB! What about A and B |
|
Mixing and Potential Energy - University of Waterloo
schematic of mixing and the total energy bud-get The salient point here is that stirring is a reversible process between kinetic and potential energy whereals dissipation di?usion and mixing are irreversible (and thus one-way) processes This means that the process of mixing permanently re-moves kinetic energy from the system increasing |
|
Chapter 2 Thermodynamics of Combustion - NRC
The average intrinsic properties of a mixture can be classi?ed using either a molar base or a mass base For instance the internal energy per unit mass of a mixture u is determined by summing the internal energy per unit mass for each species weighted by the mass fraction of the species u ¼ U m ¼ P i m iu i m ¼ X i y iu |
|
Searches related to internal energy of mixing filetype:pdf
mixing there are ? possible states using the Boltzman expression for entropy we have Sterling’s approximation can be used to simplify this expression so where x 1 is the mole fraction of component 1 For an ideal gas system with no enthalpic interaction this is the free energy for mixing ?G=-T?S We can consier a probability p(x |
What is the difference between Gibbs energy and thermodynamic potential?
- is the Helmholtz energy, g is the Gibbs energy, v is the volume, T e temperature,is th and p is the pressure. Thermodynamic potentials are equivalent because they are Legendre-transforms of each other. The two potentials with measurable quantities as independent variables are the Gibbs energy g and the Helmholtz energy a
Are there other mixing rules for the mixture covolume B M?
- (16) Other mixing rules have been proposed for the mixture covolume b m , including quadratic mixing rules (see for instance McFarlane et al. [26]). The extension of the derivatives of
What are the advantages of the mixture model?
- This model has the advantage that highly accurate formulations for pure fluids can be directly used in the mixture model; all pure fluid contributions to the reduced residual Helmholtz energy are evaluated at the same reduced temperature ? and reduced density ?, not at the same temperature
How is the change of enthalpy of an ideal gas described?
- For thermodynamic systems without chemical reactions, the change of enthalpy of an ideal gas is described by the sensible enthalpy, 24 2 Thermodynamics of Combustion h^ si¼ ZT T o ^c pðTÞdT; wherethesubscriptireferstospeciesi,T
|
Mixtures
In general, the properties of one component will depend on the presence of all other components For instance, the internal energy of component α will depend on temperature T and total pressure p of the mixture, and on all mole fractions Xβ, β = 1, ,ν, that is ¯uα = ¯uα (T,p,Xβ) |
|
Part 3 Thermodynamics of Mixing Liquids
The partial molar volume of component J in a mixture is the volume change in The chemical potential in terms of each of the internal energy U , the enthalpy H |
|
Chapter 8 Thermodynamic Properties of Mixtures
29 mar 2012 · where is the molar internal energy, is (8 the internal ener ) g 1-1 Summation of the proper before mixing at ties of pure fluids ( ) and C |
|
Part I : Preliminaries (Thermodynamics and Kinetics - NPTEL
Let us consider the calculation of these two quantities, namely, internal energy ox mixing and entropy of mixing as a function of composition 3 2 1 Congurational |
|
CHAPTER 3 THERMODYNAMICS OF DILUTE GASES - Stanford
1 avr 2013 · The internal energy of a system is determined by its temperature and volume Figure 3 9 Thermal mixing of an ideal gas at two temperatures |
|
Chapter 5: The Thermodynamic Description of Mixtures
Partial molar quantities • Volume • Gibbs Energy (Chemical Potential) • Gibbs- Duhem Equation • Thermodynamics of Mixing • Henry's Law • Raoult's Law |
|
(Lec 3 Solution Models)
Molar Gibbs free energy of mixing ∆HM Molar enthalpy of mixing ∆SM Molar entropy of mixing ∆eG Excess Gibbs free energy per mole of solution ∆eH |
|
Thermodynamics and Phase Diagrams - Sistemas EEL
3 fév 2012 · resents the difference in internal energy, E, before and after mixing The difference in entropy between the mixed and unmixed states is ∆Smix |
|
Binary Solutions
free energy caused by the mixing mix mix mix energy change upon mixing is only due to the change in Let's calculate the internal energy of the solution: AB |