medical device regulation singapore
|
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010
certificate of origin certifying that the medical device is manufactured in Singapore and registered under the Act. (3) An application for a certificate |
|
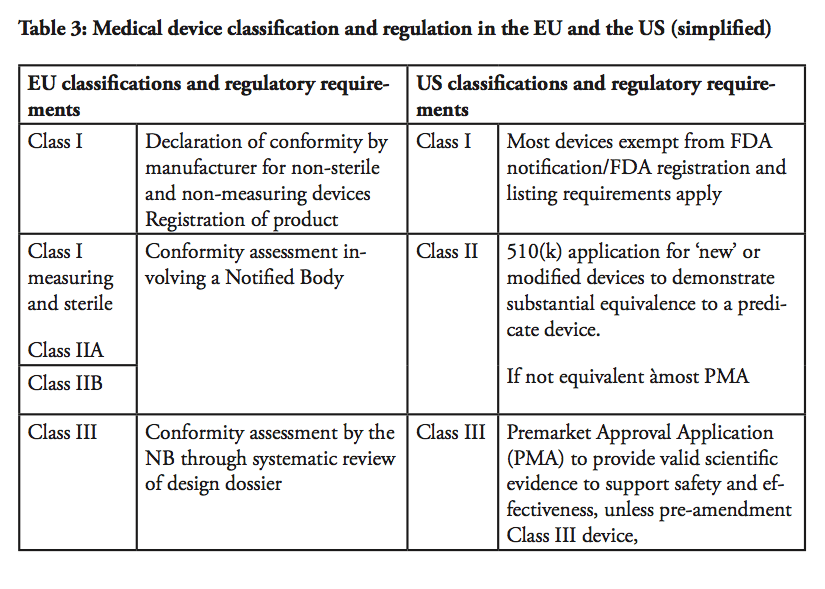
The EUSFTA and Beyond: Trade in Pharmaceutical Products and
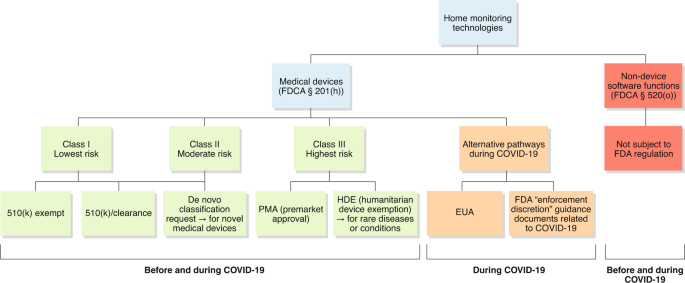
There are 4 classes of medical devices classes A |
|
GN-32: Guidance for Importation of Unregistered Medical Devices
1 sept. 2022 Such products will not be subject to medical device regulatory controls in Singapore and hence are excluded from the scope of this guidance note ... |
|
Regulatory Guidelines for Software Medical Devices – A Lifecycle
submitted for device registration in Singapore. 298. 299. The essential design and manufacturing principles that may be relevant to software medical devices. |
|
Medical Devices - Baker McKenzie
In August 2010 Singapore implemented the Health Products (Medical. Devices) Regulations 2010 |
|
GN-15: Guidance on Medical Device Product Registration
7 janv. 2022 device under the Health Products Act the supply and use of any medical device in Singapore should also comply with the requirements under ... |
|
Medical-devices-product-classification-guide.pdf
some countries it will also be a medical device in Singapore. This is not the case and Product |
|
GN-15: Guidance on Medical Device Product Registration
5 août 2021 device under the Health Products Act the supply and use of any medical device in Singapore should also comply with the requirements under ... |
|
Regulatory Guidelines for Software Medical Devices – A Life Cycle
29 avr. 2022 submitted for device registration in Singapore. The essential design and manufacturing principles that may be relevant to software medical ... |
|
ASEAN Medical Devices Regulation
The Association of Southeast Asian Nations (ASEAN) represents a group of 10 countries in Southeast Asia comprising Indonesia Singapore |
|
November 2022 - MEDICAL DEVICE GUIDANCE
3 nov 2022 · This document provides guidance to assist a product owner in the preparation of a Declaration of Conformity (DOC) to Singapore's regulatory |
|
GN-33 - MEDICAL DEVICE GUIDANCE
1 nov 2022 · This document is intended to provide general guidance to SS 620: 2016 Singapore Standard for Good Distribution Practice for Medical Devices |
|
Medical Devices - Baker McKenzie
Medical Devices In August 2010 Singapore implemented the Health Products (Medical Devices) Regulations 2010 pursuant to the Health Products Act |
|
Health Products (Medical Devices) Regulations 2010 - Singapore
1 These Regulations may be cited as the Health Products (Medical Devices) Regulations 2010 and shall come into operation on 10th August 2010 |
|
Regulatory Updates Health Sciences Authority Singapore
Regulatory Updates Health Sciences Authority Singapore September 2022 Dr Rama Sethuraman Director Medical Devices Medical Devices Cluster |
|
Regulatory Updates Health Sciences Authority Singapore
* Class A and devices incorporating registrable medicinal/ therapeutic products are not eligible for the Priority Review Scheme Falls under 1 of the 5 |
|
Singapore Medical Devices Regulatory Updates
Health Products (Medical Devices) Regulations 2010 • Hierarchy of regulatory requirements ? Guidance Documents ( Requirements available on the web |
|
Regulatory Updates on Singapore Medical Device Market
Registration of Class C and D medical devices begin Applications for product registration must be submitted by the start of Phase 3A on August 10 2010 ? |
|
Trade in Pharmaceuticals and Medical Devices under the EUSFTA
21 fév 2022 · Between the EU and Singapore medicaments medicinal products and pharmaceutical products accounted for over 6 billion Euros of trade – some of |
|
ASEAN MEDICAL DEVICE DIRECTIVE
HAVING regard to the objectives of harmonised medical device regulations common technical documents and the progress made in implementation |
How are medical devices regulated in Singapore?
In Singapore, medium- and high-risk medical and in vitro diagnostic (IVD) devices must be registered with the Health Sciences Authority (HSA). Low-risk devices are not registered; they are instead listed on the local importer's license in the HSA's online system.Who regulates medical devices in Singapore?
Medical devices in Singapore are regulated by the Medical Device Branch of the Health Sciences Authority (HSA). Singapore is a member of the Association of Southeast Asian Nations (ASEAN) and its regulatory system is based on the Health Products Act 2007 and Health Products (Medical Devices) Regulations 2010.What is the new medical device regulation?
The new MDR regulation implies that it is no longer possible to market medical devices that contain or consist of viable biological material or viable organisms, including living micro-organisms, bacteria, fungi or viruses in order to achieve or support the intended purpose of the product.- Medical devices are health products which have a physical or mechanical effect when used on human bodies.
|
Singapore - WHO World Health Organization
html For the registration of a medical device, it is required that it is: safe to use, of suitable quality as to its specifications, manufacture, and measures taken to ensure that quality, effective for its intended purpose Health Products Act (Medical Devices) Regulations, 25 |
|
Regulatory Guidelines for Software Medical Devices - Health
submitted for device registration in Singapore 298 299 The essential design and manufacturing principles that may be relevant to software medical devices |
|
MEDICAL DEVICE GUIDANCE - Health Sciences Authority
3 avr 2019 · GN-15: Guidance on Medical Device Product Registration Revision 7 3 supplying the health product by export to a party outside Singapore |
|
Medical Devices - Baker McKenzie
Singapore Medical Devices In August 2010, Singapore implemented the Health Products (Medical Devices) Regulations 2010, pursuant to the Health |
|
Regulatory Updates on Singapore Medical Device Market
Responsible for the regulation of medical devices, drugs, national blood bank, and healthcare services ▫ www hsa gov sg Centre for Medical Device Regulation |
|
HEALTH PRODUCTS (MEDICAL DEVICES) REGULATIONS 2010
certificate of origin certifying that the medical device is manufactured in Singapore and registered under the Act (3) An application for a certificate under |
|
HSA Circular to Exhibitors and Importers
In Singapore, medical devices are subject to regulation under the Health Products Act Under this law, the import and supply of all medical devices, including in |
|
COVID-19 Medical Devices: Singapore - Bird & Bird
Due to the COVID-19 pandemic, many countries have enacted emergency legislation removing certain regulatory barriers and exempting products from |
|
MEDICAL DEVICE GUIDANCE - Globi-Reg
Medical Device Branch Health Products Regulation Group Health Sciences Authority 11 Biopolis Way #11-01 Helios Singapore 138667 Fax: (65) 6478 9028 |
|
ASEAN Agreement on Medical Device Directive - ASEAN Legal
Singapore, the Kingdom of Thailand and the Socialist Republic of Viet Nam, Member Medical Device Directive (hereinafter referred to as “Agreement") and its |










/tuv-rheinland-ivdr-visual-1-en.png)