naoh hydrolysis of ester
|
Efficiency of lithium cations in hydrolysis reactions of esters in
٣١/٠٣/٢٠٢١ Ethyl benzoates were hydrolyzed using hydroxides (KOH NaOH |
|
Mild alkaline hydrolysis of hindered esters in non-aqueous solution
Typical methods for saponification involve the use of aqueous solutions of water-miscible solvents large excesses of hydroxides |
|
Chapter 5 Carboxylic Acids and Esters
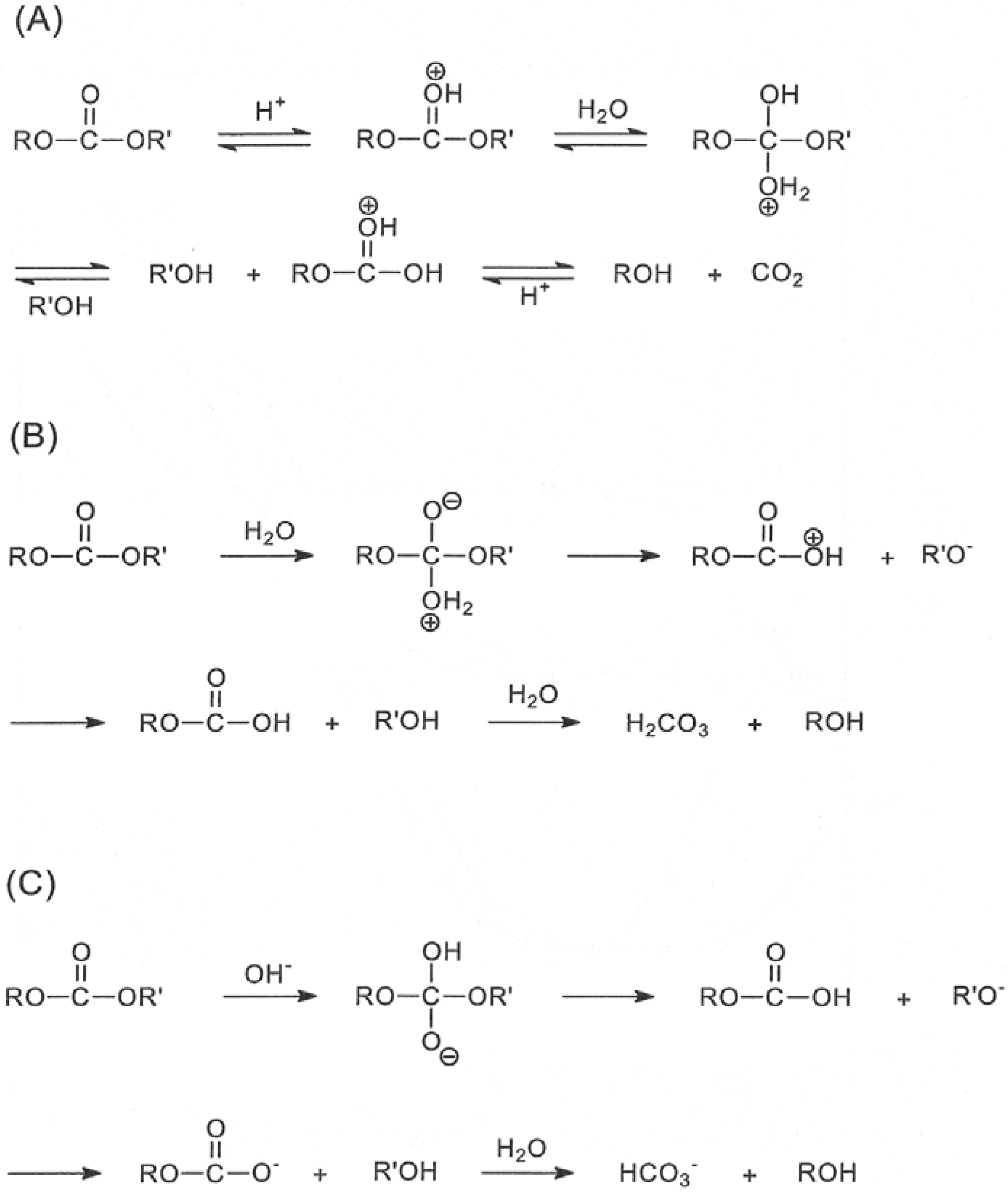
Reactions of Esters: Ester Hydrolysis. • Esters may be broken apart under Saponification of an ester to produce a carboxylate salt and an alcohol. R. C. O. O. |
|
Solvent Effects and Ester Interchange in Basic Hydrolysis of Esters
The saponification of ethyl acetate and methyl acetate has been measured at 30° in dioxane-water mixtures containing additional solvents found earlier to |
|
TOPIC: ORGANIC CHEMISTRY 8 – Base hydrolysis of an ester
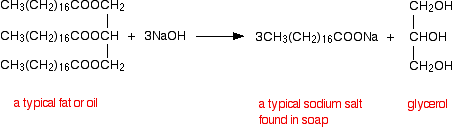
Soap is made when fats which are natural esters are heated with bases such as potassium hydroxide or sodium hydroxide (potassium hydroxide is more soluble in |
|
Scope and Limitations of Sodium and Potassium Trimethylsilanolate
4 Besides saponification esters can be hydrolyzed with various protic or tion and acid-catalyzed hydrolysis of esters can be limited in the cases of ... |
|
Mild alkaline hydrolysis of hindered esters in non-aqueous solution
٢٧/١٠/٢٠١٨ Alkaline hydrolysis of an ester is one of the most extensively investigated reactions. Typical methods for saponification involve the use of ... |
|
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
In this hydrolysis of ester (ethyl acetate) with an alkali (sodium hydroxide) HCl was used as catalyst to accelerate it. 1ml and 2ml of ethyl acetate was |
|
TO STUDY THE KINETICS OF SAPONIFICATION OF AN ESTER BY
Hence alkaline hydrolysis of esters came to be known as saponification. You have learnt about the integrated rate equation for a second order reaction where the. |
|
Efficiency of Lithium Cations in Hydrolysis Reactions of Esters in
hydrolysis of esters with hydroxides (KOH NaOH |
|
Chapter 5 Carboxylic Acids and Esters
predict the products of ester synthesis and hydrolysis reactions. + NaOH. H2O. O. O carboxylic acid base metal carboxylate. CH3CH2CH2CH2. |
|
Efficiency of lithium cations in hydrolysis reactions of esters in
31-Mar-2021 Ethyl benzoates were hydrolyzed using hydroxides (KOH NaOH |
|
IV SEMMESTER
The Rate Constant for the hydrolysis of an ester from. 1. Calculated value = 2. Graphical value = S.No. Time. Min. Volume of. NaOH. |
|
Mild alkaline hydrolysis of hindered esters in non-aqueous solution
27-Oct-2018 Typical methods for saponification involve the use of aqueous solutions of water-miscible solvents large excesses of hydroxides |
|
Determination of the relative rates of alkaline hydrolysis of esters by
The alkaline hydrolysis of an ester (saponification) is an irreversible reaction which normally takesplace by a BAC2 mechanism1. |
|
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
In this hydrolysis of ester (ethyl acetate) with an alkali (sodium hydroxide) HCl was used as catalyst to accelerate it. 1ml and 2ml of ethyl acetate was |
|
Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green
27-Apr-2022 Since. SPPS using the Fmoc strategy relies on the activation of the carboxylic acid saponification is a mandatory step. Ester hydrolysis is a ... |
|
21.7 HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES
age reaction with water) to yield carboxylic acids. A. Hydrolysis of Esters. Saponification of Esters One of the most important reactions of esters is the |
|
Chem 360 Jasperse Ch. 20 21 Notes + Answers. Carboxylic Acids
b) “Lateral” hydrolysis: From esters with water and acid catalysis (ACID Since the reaction with NaOH is always downhill all of these reactions work. |
|
Solvent Effects and Ester Interchange in Basic Hydrolysis of Esters
The saponification of ethyl acetate and methyl acetate has been measured at 30° in dioxane-water mixtures containing additional solvents found earlier to |
|
Esters
Hydrolysis of esters in basic media provides a carboxylate salt and an alcohol which when acidified provides a carboxylic acid and an alcohol Unlike the acid |
|
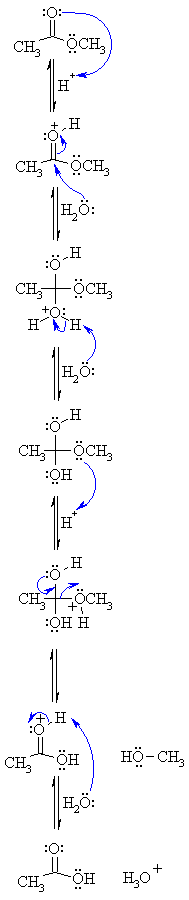
159: Hydrolysis of Esters - Chemistry LibreTexts
31 jan 2022 · Write an equation for the acidic hydrolysis of methyl butanoate and name the products When a base (such as sodium hydroxide [NaOH] or potassium |
|
Kinetics of alkaline hydrolysis of the monomethyl ester of
In this paper the results of measurements of the rate and activation parameters of alkaline hydrolysis of the monomethyl ester of terephthalic acid in mixed |
|
Kinetics of fast alkaline hydrolysis of esters - ScienceDirectcom
The experiments were carried out in stirred cells of known interfacial area containing an aqueous solution of sodium hydroxide and the ester satur- The rate of |
|
Solvent Effects and Ester Interchange in Basic Hydrolysis of Esters
The saponification of ethyl acetate and methyl acetate has been measured at 30° in of Hydrolysis of Ethyl Acetate at 30° in 0 048 M NaOH in |
|
Kinetics of alkaline hydrolysis of synthetic organic esters - ChemRxiv
The alkaline hydrolysis of esters is known to follow second-order kinetics—first-order with respect to each of the reactants the ester and OH- 15 Because OH- |
|
A simple method for the alkaline hydrolysis of esters - ResearchGate
Request PDF A simple method for the alkaline hydrolysis of esters A very mild and rapid procedure for the efficient alkaline hydrolysis of esters in |
|
Chapter 5 Carboxylic Acids and Esters - Angelo State University
Learn the major chemical reaction of carboxylic acids and esters and learn how to predict the products of ester synthesis and hydrolysis reactions |
|
Basic Hydrolysis of Esters - Saponification - Master Organic Chemistry
27 oct 2022 · Esters can be hydrolyzed to give carboxylic acids with hydroxide ion a process called saponification Most famously used for making soap |
What is the hydrolysis of an ester with NaOH?
Ester Hydrolysis with NaOH or base catalysed ester hydrolysis is the reaction of an ester with water under a basic medium. In it, an ester is heated under reflux with dilute NaOH to yield carboxylate salt and alcohol. It is also known as saponification reaction, i.e. it is used to synthesise soap.Why sodium hydroxide is preferred in hydrolysis of esters?
This is the usual way of hydrolysing esters. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution. There are two big advantages of doing this rather than using a dilute acid. The reactions are one-way rather than reversible, and the products are easier to separate.Can NaOH be used for hydrolysis?
Sodium hydroxide reacts readily with carboxylic acids to form their salts, and it is even a strong enough base to form salts with phenols. NaOH can also be used for the base-driven hydrolysis of esters (as is saponification), amides and alkyl halides.- CONCLUSION. From the above tests it is concluded that ethyl acetate hydrolyses into ethanol and ethanoic acid with a faster rate due to the catalystic effect of NaOH (base) solution, unless the process takes hours to complete the reaction.
|
IV SEMMESTER
TABULATION: RESULT: The Rate Constant for the hydrolysis of an ester from 1 Calculated value = 2 Graphical value = S No Time Min Volume of NaOH ml |
|
Hydrolysis of ester lab report - Squarespace
Experiment 6: Chemical equilibrium — Hydrolysis of ethyl acetate targets: ü Sample calculation: The volume of 1,090 M NaOH used for titration was: Vials 1 |
|
Kinetics of fast alkaline hydrolysis of esters - ScienceDirectcom
The amount of any ester in water and in any aqueous solution was found by the saponification method The rate of alkaline hydrolysis was followed by noting the |
|
Facile Hydrolysis of Esters with KOH-Methanol at Ambient
27 nov 2003 · Ester hydrolysis has been known for long and is usually catalyzed by acids and bases Both acidic and alkaline hydrolysis are equilibrium |
|
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
In this hydrolysis of ester (ethyl acetate) with an alkali (sodium hydroxide), HCl 250ml conical flask containing 0 05N HCl, and titrated against 0 05N NaOH |
|
Experiment C: Hydrolysis of a Carboxylic Acid Ester:
the transformation of organic esters in aquatic environments is hydrolysis a constant ionic strength using a 1:1:1 mix of 3M KCl/buffer or NaOH solution/10-4 M |













![PDF] The Alkaline Hydrolysis of Sulfonate Esters: Challenges in PDF] The Alkaline Hydrolysis of Sulfonate Esters: Challenges in](https://upload.wikimedia.org/wikipedia/commons/thumb/f/f3/Ester_hydrolysis.png/400px-Ester_hydrolysis.png)