nh4 pka
|
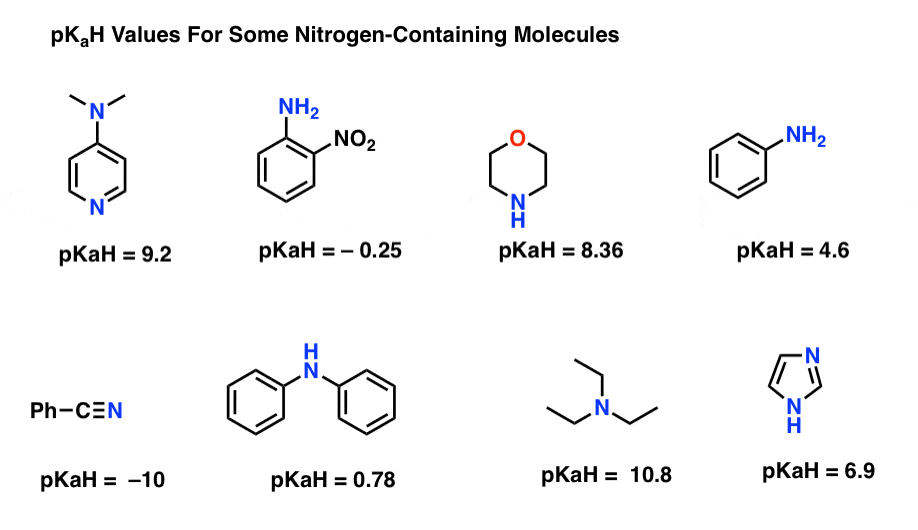
Amine Basicity of Amine NH2 < N H N < Ammonium pKa NH2 NH4
NH4. +. NH4+. NH3. +. NH2. +. NH+. 5.2. 5.2. 0.4. 9.26. 10.8. 11.3 higher pKa higher basicity |
|
Zuren en basen
1200 mL werd toegevoegd? pKa (HOAc) = 4 |
|
Zuren en basen
pKa (HOAc) = 475. pKb (NH3) = 4 |
|
From Analytical to Prep
Ammonium Formate pKa 2. 9.20. 8.2-10.2. NH4COOH. NH4. + ↔ NH3. 0.64 g. HCOOH or NH4OH. Triethylammonium Acetate (TEAA) pKa 1. 4.76. 3.8-5.8. (CH3CH2)3N: |
|
Acids and Basesx
(aq) + OH– (aq) Kb = [NH4. +][OH–] / [NH3]. For convenience the values are usually recorded as pKa or pKb values where pKa = -log Ka. In fact all acids with a ... |
|
Chemical profile for Ammonium sulfide
(NH4)2S (s) → 2[NH4]+. (aq) + S2-. (aq). However very little S2- is present in solution as the S2- ion is very basic (Kb = 1 x 105). For water pKa = 15.7 and |
|
BUFFEROPLOSSINGEN Inleiding Zowel in de analytische chemie
Bereken de pH van de oplossing die ontstaat als we 1 mol NH3 en 1 mol NH4Cl per. 500 ml oplossen. Uitwerking: NH4. + + H2O ↔ NH3 + H3O+. We passen de |
|
7 6 6 ( ) ) 7 3984 : 8 17 : . - B ( ) 7 3984 : 8
pKa = “–7”. pKa = 4.8. Page 13. 1 lC. 1. pKa = 3.8. pKa = 4.8. pKa = 0.2. Page 14. pK [NH4. +. ] = 10. –9.4. . K = [CH3. COO. –. ][NH4. +. ] [CH3. COOH][NH3. |
|
Bipolar Membrane Electrodialysis for Ammonia Recovery from
pKa = 9.25; % dissociation constant of NH4. +. 85. kH = 1631.3; % Pa/M Henry constant of NH3. 86. D_salt = [1.33 |
|
. Dra. Patrícia Bulegon Brondani (@patyqmc) Resolução da lista de
+ = 94 |
|
. Dra. Patrícia Bulegon Brondani (@patyqmc) Resolução da lista de
está indicada corretamente (dados pKa do NH4. + = 94 |
|
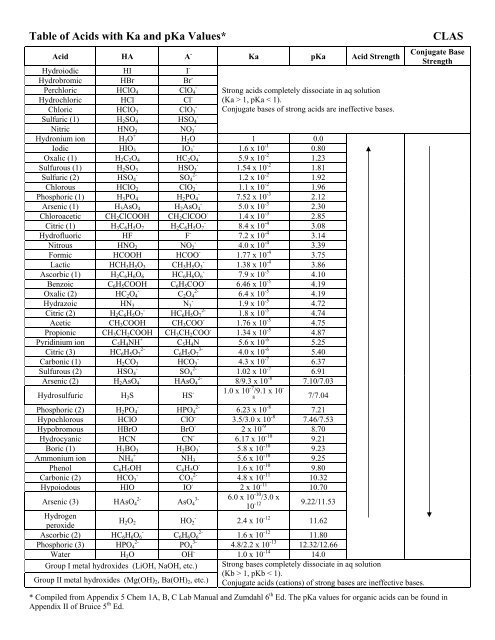
Table of Acids with Ka and pKa Values* CLAS
%20Base%20Strength/Table%20of%20Acids%20w%20Kas%20and%20pKas.pdf |
|
QMC 5222: Química Orgânica A Professor: Antônio Luiz Braga
Explique. O ácido mais forte é aquele que possui o menor pKa e sua base conjugada mais fraca. Sendo assim NH4. |
|
Equilíbrio Ácido-Base - Exercícios Resolvidos
equilíbrio. b) Calcule o pKa de cada ácido. c) Qual o ácido mais forte e qual o mais fraco? Justifique a sua resposta. d) Qual dos três ácidos HF |
|
Ácidos & Bases
C) +NH4. D) -NH2. E) +NH2. 9) Indicar o ácido e a base conjugada de HSO4-. C) NH4+. D) NH2-. 13) Qual o pH de uma solução 0.1 M de HCl? (Nota: pKa do ... |
|
2. Reacções ácido-base.
a da espécie NH4+ é baixa; ou seja a base NH3 é uma base fraca. ?log (Ka Kb) = ?log Ka ?log Kb = pKa + Kb = pKw = 14. |
|
. Dra. Patrícia Bulegon Brondani (@patyqmc) Lista de Exercícios 3
está indicada corretamente (dados pKa do NH4. + = 94 |
|
Amine Basicity of Amine NH2 < N H N < Ammonium pKa NH2 NH4
NH4+. NH3. +. NH2. +. NH+. 5.2. 5.2. 0.4. 9.26. 10.8. 11.3 higher pKa higher basicity |
|
QUIO94 - Introdução à Análise Química TITULAÇÃO ÁCIDO-BASE
BC: [NH4. +] = [OH. -] = x. BM: Ca = [NH4OH] + [NH4. +] a)Se Ca/Kb > 102 Alaranjado de metila e carmim índigo (pKa = 37). (indicador) (corante azul). |
|
EQUILÍBRIOS ÁCIDO-BASE
+ H2O ? NH4. +. + OH. -. Base 1 Ácido 2. Ácido 1. Base 2 onde a amônia NH3 (base 1) e o íon amônio |
|
Table of Acids with Ka and pKa Values* CLAS - UC Santa Barbara
%20Base%20Strength/Table%20of%20Acids%20w%20Kas%20and%20pKas.pdf |
|
PKa Chart 1 2 conjugate acid conjugate base conjugate acid
H2SO4 HBr HI I Br HSO4 TsOH HNO3 HF O H O H O H H O H O H H O H O O O H NH H2CO3 HN 3 O H H H2S HCl Cl H F N NO3 SH TsO- HCO3 N O O-10-9-8-3 6-2 4-1 7-1 3 4 7 4 8 3 2 sulfuric acid hydroiodic acid hydrobromic acid |
|
Trans- [PtCl2 (NH3)2] Transplatin D2h
pKa Data Compiled by R Williams pKa Values INDEX Inorganic 2 Phenazine 24 Phosphates 3 Pyridine 25 Carboxylic acids 4 8 Pyrazine 26 Aliphatic 4 8 Aromatic 7 8 Quinoline 27 Phenols 9 Quinazoline 27 Alcohols and oxygen acids 10 11 Quinoxaline 27 Amino Acids 12 Special Nitrogen Compounds 28 Peptides 13 Hydroxylamines 28 Nitrogen Compounds 14 |
|
Buffer pKa and pH Range Values - University of Nebraska–Lincoln
Buffer pKa and pH Range Values For preparation of Buffers in the pH Buffers pKa range Hydrochloric Acid - HCl 0-2 Nitric Acid - HNO 3 Perchloric Acid – HClO 4 Potassium Chloride – KCl 1 1-1 8 Oxalic Acid – C 2 H 2 O 4 |
|
PKa's of Inorganic and Oxo-Acids Chem 206
pKa's of CH bonds in Hydrocarbons and Carbonyl Compounds Me Me MeMe Me O X O EtEt S i-Pr O O t-BuMe Ph X Phi-Pr O O LiO O Ph Me O X O O O O O MeMe t-BuO O t-BuOMe O Ph EtO O N+Me 3 O EtO Me O O MeO OMe O O MeO S N+Me 3 O Et 2N Ph O Me 2N Me 2N O SPh N O CN Me 2NMe S O Me 2N Me O Chem206 For a comprehensive compilation of Bordwell pKa data see |
|
Searches related to nh4 pka filetype:pdf
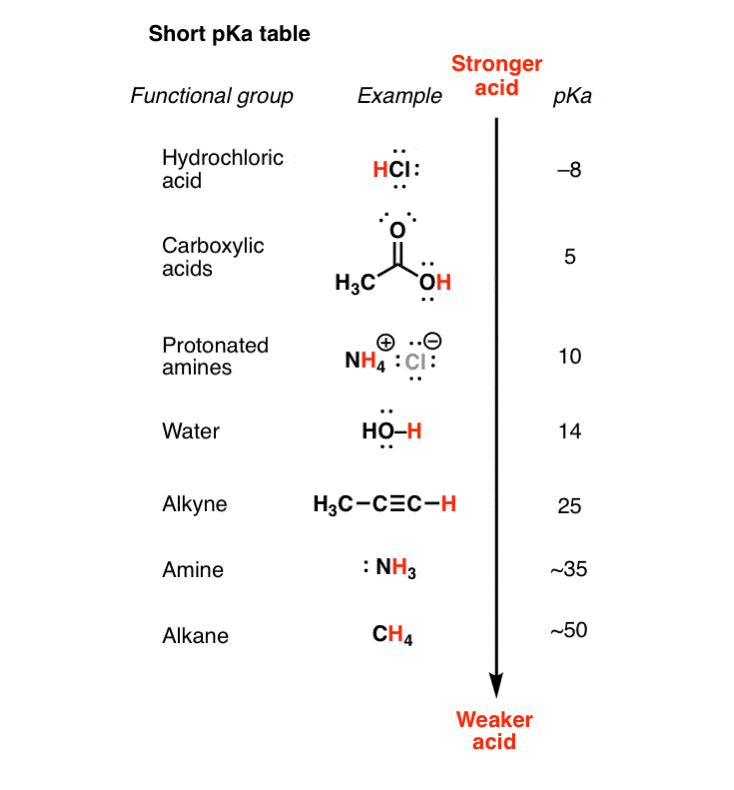
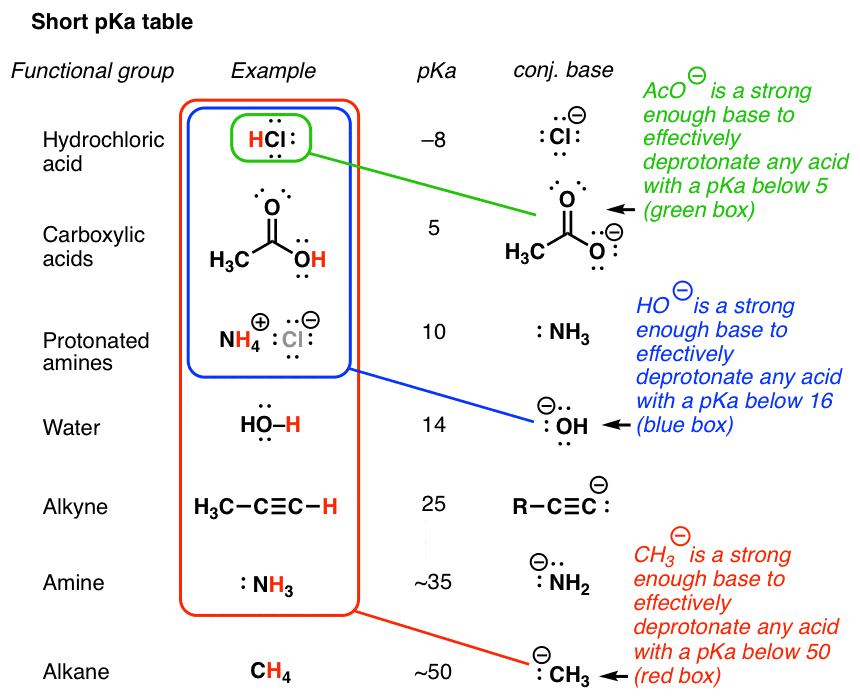
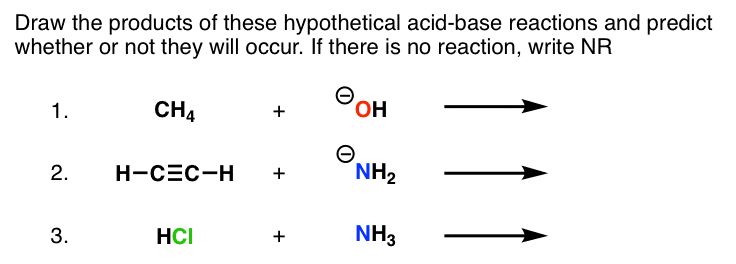
pKa Table: Effect of electronegativity and resonance Acid pKaConjugate Base HCl -7 Cl- H2SO4 -4 HSO4- HNO3 -2 NO3 H CH3CH2OH-2 CH3CH2OH ity idHO cH -2 H2O OOH 5 O H HNH OH phenol OO OEtacetoacetic ester HO-H water CH3CH2OH ethanol HH HHHacetone CCHalkyne H2N-H ammonia H-HHygrogen 9 (10)H3N 10 ONa+ phenoxide e g sodium phenoxide |
What is the point group of NH3?
- trans- [PtCl 2 (NH 3 )] contains a C 2 main rotation axis, two orthogonal C 2 axes and three ? v planes. It also has an inversion centre. Hence it belongs to the D 2h point group. This representation ignores the orientation of hydrogen on ammonia.
What is the valency of NH3?
- The formula of ammonia NH3 and it is a compound. Its valency is 0 as it follows the Octet rule that is 5 + 3 = 8 electrons in the outermost orbit. Its oxidation number is equal to zero, which means it is a very stable compound, it does not lose or gain any more electrons. In terms of ammonium ion, its valency is +1.
What would the hybridization of NH3 be?
- What is the hybridization of nitrogen in ammonia nh3 )? The nitrogen atom in NH3 is sp3 hybridized. If we consider the Lewis structure of ammonia, the four electron pairs around the nitrogen atom require a tetrahedral arrangement. The tetrahedral set of sp3 is obtained by combining the 2s and three 2p orbitals.
What is the full name of NH3?
- The name of the compound NH3 is ammonia, other names are azane, tri hydrido nitrogen, nitrogen trihydride. It is a colourless gas with a characteristic pungent smell, it is alkaline in nature. Its molar mass is 17 g/mol, melting point is -77 degree Celcius, the boiling point is - 33 degree Celcius.
|
PKa de divers couples acido-basiques - Forums Futura-Sciences
Base conjuguée Nom de la base conjuguée pKa HClO4 acide perchlorique HCN acide cyanhydrique CN- ion cyanure 9 2 NH4 + ion ammonium NH3 |
|
Chapitre 1 Acides et bases
1 1 1) et pKa dans lesquels l'argument du log est bien sans 8 CHAPITRE 1 ACIDES ET BASES 2) Ammonium / ammoniaque : NH4 + pKa = 9, 2 NH3 pH |
|
PH dune solution de chlorure dammonium pH = 56 - Umtice
pKa (NH4 +/NH3) = 9 25 Le chlorure d'ammonium NH4Cl est un sel soluble un acide faible ; il va rester de façon prédominante sous cette forme (pH 〈 pKa) |
|
Transformations chimiques en solution aqueuse - Chimie en PCSI
CLASSEMENT DES ACIDES ET DES BASES SUR UNE ECHELLE DE PKA 10 4 indifférente : c'est la base faible conjuguée de l'ion ammonium NH4 +) |
|
Chap 6 acide-base
+ ]éq / [CH3-COOH]éq pKA = 4,80 et KA = 1,58 10 -5 * Couple ion ammonium / ammoniac : équation de la réaction avec l'eau : NH4 + + H2O = NH3 + H3O + |
|
1 Réactions acido-basiques
On donne : pKa(NH4+/NH3) = 9,25 Quel volume de la solution d'acide faut-il ajouter ? AD 2 Mise en solution d'un acide dans l' |
|
PH et pKa - Eli Zysman-Colman
Plus la valeur de pKa est faible, plus le Ka est grand, plus l'acide est fort 4 38 25 10 x 1 10 10 = = - - Keq NH3 NH4 + NH2 - pKa = 9 2 pKa = 38 H H |
|
Table of Acids with Ka and pKa Values* CLAS - UCSB CLAS
pKa Acid Strength Conjugate Base Strength Hydroiodic HI I- Hydrobromic NH4 + NH3 5 6 x 10-10 9 25 Phenol C6H5OH C6H5O- 1 6 x 10-10 9 80 |



![pKa Value - [PDF Document] pKa Value - [PDF Document]](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/5-amine-basicity-is-measured-by-examining-pka-of-the-conjugate-acid-pka-of-pyridinium-is-5-pka-of-piperidinium-is-11.gif)




















![Tabla de Ka y pKa - [PDF Document] Tabla de Ka y pKa - [PDF Document]](https://0.academia-photos.com/attachment_thumbnails/43184911/mini_magick20190216-20996-1khhp0b.png?1550347672)












![How To Use A Pka Table — Master Organic Chemistrypdf [wl1p1dj979lj] How To Use A Pka Table — Master Organic Chemistrypdf [wl1p1dj979lj]](https://docplayer.net/docs-images/47/20852334/images/page_3.jpg)












![Acid and Base Titrations - [PDF Document] Acid and Base Titrations - [PDF Document]](https://i1.rgstatic.net/publication/340865553_Anti-Melanogenic_Effect_of_Dendropanax_morbiferus_and_Its_Active_Components_via_Protein_Kinase_ACyclic_Adenosine_Monophosphate-Responsive_Binding_Protein-_and_p38_Mitogen-Activated_Protein_Kinase-Medi/links/5ea19f55458515ec3aff944e/largepreview.png)

![How To Use A Pka Table — Master Organic Chemistrypdf [wl1p1dj979lj] How To Use A Pka Table — Master Organic Chemistrypdf [wl1p1dj979lj]](https://online.fliphtml5.com/tcbl/aloa/files/large/1.jpg)







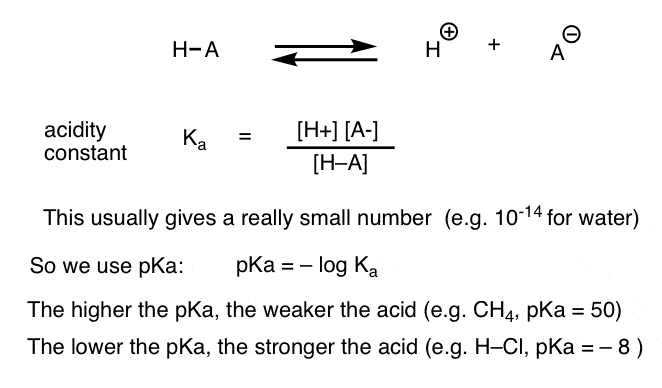


![Acid and Base Titrations - [PDF Document] Acid and Base Titrations - [PDF Document]](https://img.homeworklib.com/images/24e4f042-e790-4fbe-ac16-8adb13185676.png?x-oss-process\u003dimage/resize)


