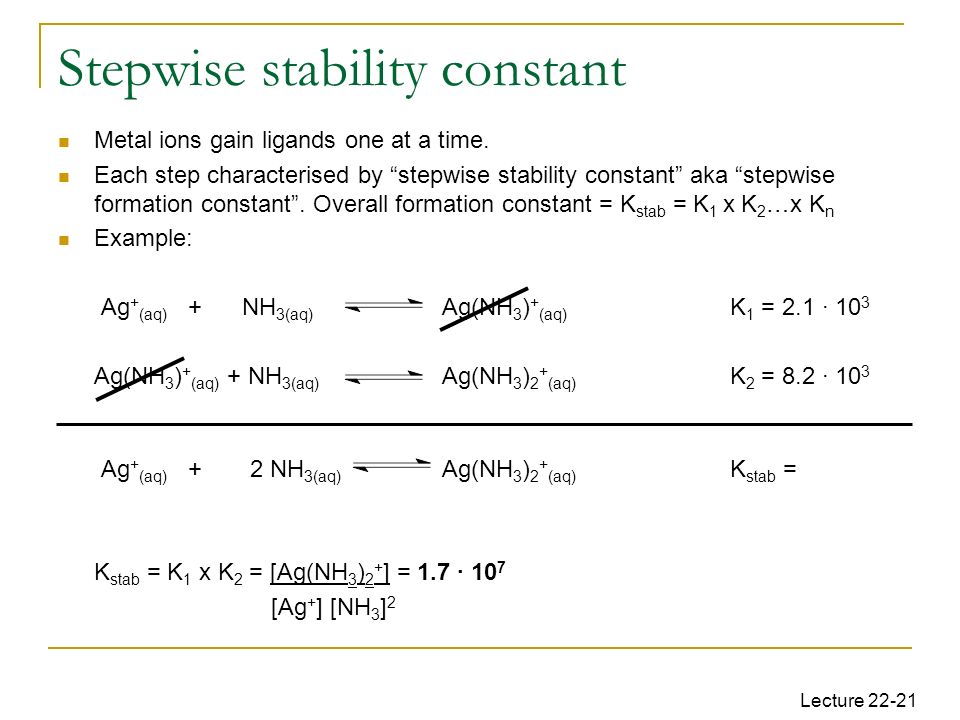
overall formation constant of cu(nh3)4 2+
|
CHEM1102 2013-N-6 November 2013 • What is the solubility of Cu
6 de nov. de 2013 (ii) the Cu2+(aq) ions that form being complexed by ammonia. For the formation of [Cu(NH3)4]2+ the equilibrium constant is: Kstab = [ ( ... |
|
Stepwise and Overall Formation Constants and Their Interactions
The formation constant or stability constant may be defined as the equilibrium constant for the stability for [Cu(NH3)4]2+ complex. Moreover the term ... |
|
CHEM1902/4 2013-N-5 November 2013 • What is the solubility of
5 de nov. de 2013 (ii) the Cu2+(aq) ions that form being complexed by ammonia. For the formation of [Cu(NH3)4]2+ the equilibrium constant is: Kstab = [ ( ... |
|
Formation Constants of Complex Ions
Formation Constants of Complex Ions www.vaxasoftware.com. Complex ion. Kf [Cu(NH3)4]2+. 1.1 × 1013. [Zn(NH3)4]2+. 7.8 × 108. [CuBr2]–. 8.0 × 105. [Zn(OH)4]2 ... |
|
Formation Constants of Complex Ions
Cu2+ + 4 NH3 ↔ [Cu(NH3)4]2+. 1.1 x 1013. Hg2+ + 4 NH3 ↔ [Hg(NH3)4]2+. 1.8 x Complex formation constants. Prof. Dr. Thomas Jüstel. FH Münster University of ... |
|
Chem 1B Lab Manual
2+ + 2e. –. (Look up the cell potential!) the overall cell reaction is the formation constant of the tetraamminecopper(II) cation: Cu2+ + 4NH3 ⇌ Cu(NH3)4. 2+. |
|
Compostos de Coordenação. Introdução - Complexos
25 de jun. de 2018 Em solução os íons metálicos são coordenados por água ou por outros ligantes. [Cu(H2O)6]2+. + 4 NH3 → [Cu(H2O)2(NH3)4]2+ ... + L Formation ... |
|
CHEM1102 2014-J-2 June 2014 • Compounds of d-block elements
2 de jun. de 2014 The overall formation constant for [Cu(NH3)4]2+ is 1.0 × 1013. Write the equation for the reaction of Cu2+ ions with excess ammonia solution. |
|
Ensino de eletroquímica no ensino médio por meio de uma
6 de nov. de 2021 do complexo tetra(amin)cobre (II) [Cu(NH3)4]2+ |
|
CHEM1902/4 2014-N-4 November 2014 • In 2009 great excitement
4 de nov. de 2014 (ii) the Cu2+(aq) ions that form being complexed by ammonia. For the formation of [Cu(NH3)4]2+ the equilibrium constant is: Kstab = [ ( ... |
|
Stepwise and Overall Formation Constants and Their Interactions
The whole process of calculating the overall formation constant can be exemplified by taking the case of [Cu(NH3)4]2+ complex. Cu2+ + NH3. |
|
CHEM1902/4 2013-N-5 November 2013 • What is the solubility of
Nov 5 2013 The overall formation constant for [Cu(NH3)4]2+ is 1.0 × 1013. Write the equation for the reaction of Cu2+ ions with excess ammonia solution ... |
|
Honors General Chemistry Test 3 Prof. Shattuck practice
Part II. 11. Calculate the concentration of Cu2+ in a solution of 0.0100 M Cu(NH3)4. 2+ with an ammonia concentration of 0.20 M. The formation constant is: |
|
Metal-Ligand Equilibria in Solution
Stepwise and Overall Formation Constants and Their Interactions. The formation of a complex between a metal ion case of [Cu(NH3)4]2+ complex. Cu2+ + NH3. |
|
CHEM1102 2013-N-6 November 2013 • What is the solubility of Cu
Nov 6 2013 The overall formation constant for [Cu(NH3)4]2+ is 1.0 × 1013. Write the equation for the reaction of Cu2+ ions with excess ammonia solution ... |
|
CHEM1102 2014-J-2 June 2014 • Compounds of d-block elements
Jun 2 2014 The overall formation constant for [Cu(NH3)4]2+ is 1.0 × 1013. Write the equation for the reaction of Cu2+ ions with excess ammonia solution ... |
|
Electrochemical Cells
electrodes (copper and zinc) in the two solutions are connected by a wire Cu(NH3)4. 2+ + 2e- the overall cell reaction is the formation equilibrium for ... |
|
Formation Constants of Complex Ions
Complex formation constants. Prof. Dr. Thomas Jüstel Complex Ion Equilibrium. Kformation. Halide complexes ... Cu2+ + 4 NH3 ? [Cu(NH3)4]2+. 1.1 x 1013. |
|
Formation Constants of Complex Ions
Formation Constants of Complex Ions [Hg(NH3)4]2+. 1.8 × 1019. [Al(OH)4]– ... [SnF6]2–. 1.0 × 1025. [Cu(CN)4]3–. 2.0 × 1030. [Zn(CN)4]2–. 1.0 × 1018. |
|
EXPERIMENT 9
The mass action expression for this equilibrium can be written as follows; K is the formation constant of the tetraamminecopper(II) ion: Kf = [Cu(NH3)4. 2+. ]. |
|
Stepwise and Overall Formation Constants and Their Interactions
The overall stability constant is generally reported in logarithmic scale as log ? as given below log =log 1+log 2+log 3+log 4+log 5+log 6+?log(11) Or =(12) log =?log =1 The whole process of calculating the overall formation constant can be exemplified by taking the case of [Cu(NH3)4]2+ complex |
|
Formation Constant vs Equilibrium constant - CHEMISTRY
1) and the complex ion [Cu(NH 3)4]+2 remains in solution; thus a separation is achieved IV Oxidation-Reduction Reactions Oxidation reactions can also play a role in qualitative analysis In the list of ions you are testing (Ag + Cu +2 Fe +3 Cr +3 Zn +2 and Ba +2) only Cr +3 is not in its maximum oxidation state in aqueous solution |
|
Complexation Reactions and Titrations
2+ 2+ Cu (blue) + 4:NH Cu(NH) (dark blue) 334 2+ 2+ Acid Base Complex ion • When a ligand has a single complexing or donor group in its structure it is said to be unidentate (single-toothed):NH3 Cl-Co(NH 3)6 2+ CuCl 4 2-•when there are two groups it is bidentate 4 5 6 |
|
Honors General Chemistry Test 3 - Colby College
11 Calculate the concentration of Cu 2+ in a solution of 0 0100 M Cu(NH 3)42+ with an ammonia concentration of 0 20 M The formation constant is: Cu 2+ (aq) + 4 NH 3 (aq) ?? Cu(NH 3)42+ (aq) Kf = 5 0x10 12 Cu(NH 3)42+ ?? Cu 2+ + 4 NH 3 Kd = 1/K f = 2 0x10-13 initial 0 0100 0 0 200 change –x +x +4x equil 0 0100–x x 0 200+4x |
|
822 Formation Constants of Metal Complexes - WordPresscom
8 2 2 Formation Constants of Metal Complexes Each value listed in Tables 8 12 and 8 13 is the logarithm of the overall formation constant for the stability constants As an example the entries in Table 8 12 for the zinc ammine complexes represent these equilibria: Zn 2 NH 3 2 Zn(NH 3) [Zn(NH 2 3) ] [Zn][NH 2 3] Zn 2 2NH 3 Zn(NH 3) 2 |
|
Searches related to overall formation constant of cunh34 2+ filetype:pdf
Hg2+ + 4 Br- [HgBr 4]2-3 0 x 104 Cu+ + 2 I- [CuI 2]-8 0 x 108 Ag+ + 2 I-1 0 x 10 [AgI 2]- 11 Pb2+ + 4 I- [PbI 4]2-3 0 x 104 Hg2+ + 4 I- [HgI 4]2-1 9 x 1030 Ammonia complexes Ag+ + 2 NH 3 1 6 x 10 [Ag(NH 3) 2]+ 7 Zn2+ + 4 NH 3 [Zn(NH 3) 4]2+ 7 8 x 108 Cu2+ + 4 NH 3 [Cu(NH 3) 4]2+ 1 1 x 1013 Hg2+ + 4 NH 3 [Hg(NH 3) 4]2+ 1 8 x 1019 Co2+ + 6 NH 3 |
What is formation constant?
- The formation constant is the equilibrium constant for forming a coordination complex, for example: Cu 2+ + 4NH 3 --> [Cu(NH 3) 4] 2+. the formation constant is the ratio of products to reactants for this.
Why is [Cu(NH3) 4] + a colourless complex?
- Why is [Cu (NH3) 4] + a completely colourless complex, while [Cu (NH3) 4] 2+ gives intense blue colour in solution? The answer lies in the peculiarity of the arrangement of 3d and 4s electrons in transition elements.
What are the total possible coordination isomers of [Cu(NH3)4]?
- The total possible coordination isomers for the following compounds respectively are: [Co (en)3] [Cr (C2O4)3] [Cu (NH3)4] [CuCl4] [Ni (en)3] [Co (NO2)6] . ]. ] has four possible coordination isomers. ]. ] has two possible coordination isomers. They are [Cu(NH 3 )].
What happens when HCL is added to [Cu(NH3)4]2+?
- When conc. HCl is added to the solution containing [Cu (NH3)4]2+, Cl- ions will replace NH3 and form [Cu (Cl)4]2- complex. This can be understood for the following reaction: The Cl- ion is a weak field ligand, while NH3 is a strong field ligand. Hence, Cl- will in less splitting of d-orbitals, as compared to NH3.
|
Formation Constants of Complex Ions - Ars- Chemia
Formation Constants of Complex Ions at 25 °C [PbI4]2- 3 104 [HgI4]2- 1 9 1030 Ammonia complexes [Ag(NH3)2]1+ 1 6 107 [Cu(NH3)4]2+ 1 1 |
|
Stepwise and Overall Formation Constants and Their - Dalal Institute
The whole process of calculating the overall formation constant can be exemplified by taking the case of [Cu(NH3)4]2+ complex Cu2+ + NH3 1 |
|
Complex-Ion Equilibria - OMC E- library ::
H2O)(NH3)3]2+][NH3] =[[Cu(NH3)4]2+][[Cu(H2O)4]2+][NH3]4=Kf If you wanted to find the formation constant of one of the intermediate steps, you would simply |
|
Stability of Complexes: Some Elements Concerning the Kinetics of
mine) cation complex [Cu(NH3)4]2+ forms when a sufficient quantity of ammonia stants (or successive stability constants) and the overall stability constants |
|
Formation Constants of Complex Ions - VaxaSoftware
Formation Constants of Complex Ions [HgI4]2– 1 9 × 1030 [BeF4]2– 1 3 × 1013 [HgI4]2– 6 8 × 1029 [Cd(CN)4]2– 6 0 × 1018 [Ni(CN)4]2– [Cu(NH3)4]2 + |
|
Formation of complex compound pdf - Squarespace
Name the central metal ion; if the overall charge on the ligand is positive or neutral, [Ni(NH3)6]2+(aq) + 6H2O(l) The greater the stability constant, the further to the right Formation of the [Cu(NH3)4(H2O)2]2+ complex is accompanied by a |
|
Questions COMPLEX IONS: STABILITY CONSTANTS - Chemguide
b) Write an expression for the overall stability constant, Kstab, for the formation of the complex ion [Cu(NH3)4(H2O)2]2+ c) Use the values in the table for K1, K2 |
|
Chem 201--Chapter 6 lecture notes - Cal State LA
26 jan 2012 · (2) the equilibrium constant is the inverse of the forward reaction: d c b a + 4NH3(aq) → Cu(NH3)4 2+(aq) x 4x 0 125 - x Complex Formation |
|
Honors General Chemistry Test 3 Prof Shattuck, practice
Part II 11 Calculate the concentration of Cu2+ in a solution of 0 0100 M Cu(NH3) 4 2+, with an ammonia concentration of 0 20 M The formation constant is: |
|
C Aqueous Equilibrium Constants - WebAssign
Complex Ion Formation Constants in Water at 25 °C Complex Ion 1 5 * 1035 [Fe (CN)6]4- 3 * 108 [Cu(ox)2]2- 1 7 * 1013 [Cu(NH3)4]2+ 1 * 1020 [Cu(en)2]2+ |



![Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N](https://d10lpgp6xz60nq.cloudfront.net/q-thumbnail/15839883.png)
![Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N](https://i1.rgstatic.net/publication/346939099_CuSO4CuNH34SO4-Composite_Thermochemical_Energy_Storage_Materials/links/5fd324a945851568d154e359/largepreview.png)
![PDF) CuSO4/[Cu(NH3)4]SO4-Composite Thermochemical Energy Storage PDF) CuSO4/[Cu(NH3)4]SO4-Composite Thermochemical Energy Storage](https://www.chemguide.co.uk/inorganic/complexions/cunh3eqmbits.gif)



![Chem 1902 - [PDF Document] Chem 1902 - [PDF Document]](http://wwwchem.uwimona.edu.jm/courses/nichelat.gif)




2 Phase transition and dynamics of NH3 ligands in [Zn(NH3)4](ReO4)2](https://cdn.slidesharecdn.com/ss_thumbnails/sancomplexstability-161013152714-thumbnail-4.jpg?cb\u003d1476373069)







![Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N Ag+ + NH3 → [Ag(NH3)+] ; K1\u003d68 × 10-3 [Ag(NH3)+] + NH3 → [Ag(N](https://cdn.slidesharecdn.com/ss_thumbnails/chapter17complexationandprecipitationreactionsandtitrations-171212110447-thumbnail-4.jpg?cb\u003d1516274206)




