 unit-10 - preparation of organiccompounds
unit-10 - preparation of organiccompounds
(b) Melting point of acetanilide is ______ °C. Precautions. (a) Handle acetic anhydride and acetyl chloride carefully as they cause irritation to the eyes and
 Resonance March 2022 final.cdr
Resonance March 2022 final.cdr
Preparation of acetanilide by the acetylation of aniline is an important Another process for its preparation involves treating aniline with acetyl chloride ...
 MICROWAVE-AIDED REACTIONS OF ANILINE DERIVATIVES
MICROWAVE-AIDED REACTIONS OF ANILINE DERIVATIVES
compound and additionally phosphoryl chloride and hydrogen chloride. A well-known example is the synthesis of acetanilide (N-phenylacetamide) from aniline ...
 Preparation and purification of Acetanilide
Preparation and purification of Acetanilide
a) To synthesis acetanilide by reaction of aniline and acetic anhydride. b Preparation of Acetanilide. College Of Science. Chemistry Department. 2.
 Sulfa Antibiotics - Synthesis of Sulfanilamide INTORODUCTION
Sulfa Antibiotics - Synthesis of Sulfanilamide INTORODUCTION
The simplest case is the synthesis of aniline from nitrobenzene. In this reaction tin metal serves as the reducing agent and is oxidized to stannic chloride
 Amines
Amines
23-Apr-2018 Why is benzene diazonium chloride not stored and is used immediately after its preparation? 45. Why does acetylation of —NH2 group of aniline ...
 Practical Lab Manual of Pharmaceutical Organic Chemistry - I
Practical Lab Manual of Pharmaceutical Organic Chemistry - I
Acetanilide is prepared from aniline using an acetylation reaction. Acetylation is often used to place an acetyl protecting group on primary or secondary amines
 Syntheses of Medicinal Compounds
Syntheses of Medicinal Compounds
27-Sept-2017 93 g of aniline on reacting with 102 g of acetic anhydride yields acetanilide = 135.16 g ... acetic anhydride acetyl chloride
 EXPERIMENT 5 - Preparation of Acetanilide
EXPERIMENT 5 - Preparation of Acetanilide
a) To synthesis acetanilide by reaction of aniline and acetic anhydride. b) To purify acetanilide by crystallization method from water.
 Practical Lab Manual of Pharmaceutical Organic Chemistry - II
Practical Lab Manual of Pharmaceutical Organic Chemistry - II
Acetanilide is prepared from aniline when it acylating with acetic anhydride in acetyl group from acetic anhydride or acetyl chloride. Precaution. 1. As a use ...
 lelm110.pdf
lelm110.pdf
Aniline. : 5 mL. • Acetic anhydride. /Acetyl chloride method can be used for the preparation of acetanilide. Material Required. • Boiling tube.
 important exercise in organic synthesis at the undergraduate
important exercise in organic synthesis at the undergraduate
Acetylation of aniline to prepare acetanilide is part of the cur- treating aniline with acetyl chloride without using any solvent [1]. Acetyl chloride.
 Practical Lab Manual of Pharmaceutical Organic Chemistry - I
Practical Lab Manual of Pharmaceutical Organic Chemistry - I
Synthesis and Characterization of Acetanilide from Aniline by acids and bases bromine
 Studies on N-acetylation of anilines with acetyl chloride using phase
Studies on N-acetylation of anilines with acetyl chloride using phase
Keywords: Amines Acetyl Chloride
 Practical Lab Manual of Pharmaceutical Organic Chemistry - II
Practical Lab Manual of Pharmaceutical Organic Chemistry - II
Synthesis and Characterization of Acetanilide from Aniline thionyl chloride and other corrosive materials can produce severe burns and.
 Sulfa Antibiotics - Synthesis of Sulfanilamide INTORODUCTION
Sulfa Antibiotics - Synthesis of Sulfanilamide INTORODUCTION
Aniline. Acetanilide. 4-Acetamidobenzene- sulfanyl chloride. 4-Acetamidobenzene- sulfonamide. Sulfanilamide. If we have sufficient time this semester
 Synthesis of Quinoline-Thiazole Compound (Ethyle 2
Synthesis of Quinoline-Thiazole Compound (Ethyle 2
11-Sept-2020 First aniline reacts with acetyl chloride to give acetanilide. Acetanilide undergoes Vilsmeier reaction by involving use of DMF and POCl3 to ...
 An overview on the synthesis and chemical properties ofp
An overview on the synthesis and chemical properties ofp
19-Apr-2019 Acetanilides (N-acetyl aniline) are essential class of organic compounds. ... Reaction of p-aminoacetanilide 4 with different acyl chloride ...
 1138 - 23.9 aromatic substitution reactions of aniline derivatives
1138 - 23.9 aromatic substitution reactions of aniline derivatives
Outline a preparation of p-nitroaniline from aniline and any other group of aniline with acetyl chloride to give N-phenylacetamide (acetanilide) will ...
 Quantitative Acetylation of Amines by Means of Acetyl Chloride and
Quantitative Acetylation of Amines by Means of Acetyl Chloride and
was established by synthesis. were prepared. East Lansing Mich. Received July 26
 UNIT-10 PREPARATION OF ORGANIC OMPOUNDS - NCERT
UNIT-10 PREPARATION OF ORGANIC OMPOUNDS - NCERT
(i) Take 5 mL of aniline in a 100 mL round bottom flask and add acetylating mixture containing 5 mL acetic anhydride and 5 mL glacial acetic acid Alternatively you can use 5 mL of acetyl chloride and 5 mL of dry pyridine as the acctylating mixture Material Required • Funnel : One • Round bottomed flask (100 mL) : One • Beaker (250 mL
 Preparation and purification of Acetanilide - KSU
Preparation and purification of Acetanilide - KSU
Using a medicine dropper place 0 15 to 0 20 g of aniline (about 10 drops) (d = 1 02 g/ml) in a large tared test tube and determine the weight to the nearest mg Add 5 ml of distilled water to the test tube and then add 20 drops of acetic anhydride again using a medicine dropper (Fig 1) stir the mixture using stirring rod for 5 minutes until
 Unit-10 (Corrected on 24-05-08) - NCERT
Unit-10 (Corrected on 24-05-08) - NCERT
(i) Take 1 mL of aniline in a dry boiling tube add 1 mL of glacial acetic acid to it and mix the two thoroughly (ii) To the above mixture add 1 mL of acetyl chloride in lots (0 3 mL at a time) The mixture becomes warm If the boiling tube becomes unbearable to touch cool it under tap water
 Experiment 1: Synthesis of Acetamides from Aniline and
Experiment 1: Synthesis of Acetamides from Aniline and
Acetylation of aniline NH H aniline (nucleophile) CH3 O O O H3C ?-?+ acetic anhydrie (electrophile) H N CH3 O acetanilide CH3 H O + acetic acid Both aniline and acetic anhydride are somewhat viscous liquids So simply mixing them together does not result in the efficient formation of acetanilide Therefore a solvent (in this case water) is
 Tishk International University Practical Organic Chemistry II
Tishk International University Practical Organic Chemistry II
Preparation of Acetanilide Procedure 1-In a conical flask containing (60ml) of distilled water add (2ml) of concentrated hydrochloric acid (HCl) 2-Add (3ml) of aniline to the solution with shaking 3-Add (3ml) of acetic anhydride to the solution in a small portion using a burette for such addition then boil the solution to about (5min)
How to prepare acetanilide?
- • Aniline : 5 mL • Acetic anhydride /Acetyl chloride : 5 mL • Acetic acid / Pyridine : 5 mL To prepare acetanilide. The replacement of one hydrogen atom of the — NH 2 group of aniline by CH 3 CO– group in the presence of glacial acetic acid. Gives acetanilide. In the laboratory, acetylation is usually carried out with acetic anhydride.
How to mix aniline and acetyl chloride?
- (i) Take 1 mL of aniline in a dry boiling tube, add 1 mL of glacial acetic acid to it and mix the two thoroughly. (ii) To the above mixture add 1 mL of acetyl chloride in lots (0.3 mL at a time). The mixture becomes warm. If the boiling tube becomes unbearable to touch, cool it under tap water.
How do you convert aniline to acetate?
- (v) Add 9 mL of glacial acetic acid diluted with an equal volume of water and shake the reaction mixture thoroughly to convert excess aniline to its acetate, which is water-soluble. (vi) Allow the mixture to stand for 15 minutes with occasional stirring.
How do you dissolve acetanilide in glacial acetic acid?
- In a 125 mL Erlenmeyer flask, dissolve 3.4 g of acetanilide in 4 mL glacial acetic acid. You may need to warm the solution gently in order to get all the solid material to dissolve. Use your Bunsen burner for this purpose. Stir with your glass stirring rod and pass the Erlenmeyer back and forth through a low flame.
EXPERIMENT 6
Preparation of Acetanilide
College Of Science
Chemistry Department
1Preparation and purification of Acetanilide
Purpose:
a) To synthesis acetanilide by reaction of aniline and acetic anhydride. b) To purify acetanilide by crystallization method from water c) Purity check by melting rangeEquipment / Materials and Hazars:
hot plate beakers(250, 400 mL) ice stirring rod spatula Büchner funnel aniline weighing paper digital scales rubber tubing (hose) acetic anhydride filter paper Mel-temp apparatus10- mL graduated cylinder large test tube medicine dropper
Hazards FW (g/mol) MP (BP) density Compound
Irritant. Harmful
if inhaled/ingested. --- AcetanilideIrritant
(eyes/skin).Harmful if
inhaled/ingested.Possible
carcinogen.93.13 (184 ºC) 1.022 g/mL Aniline
Irritant
(eyes/skin).Toxic by
inhilation,102.09 (138 ºC) 1.082 g/mL Acetic
Anhydride
King Saud University Lap447CHEMEXPERIMENT 6
Preparation of Acetanilide
College Of Science
Chemistry Department
2Discussion:
Recrystallization is a widely-used technique to purify a solid mixture. The desired product is isolated from its impurities by differences in solubility. Insoluble impurities and colored impurities can be removed from hot solvent through the use of activated carbon and filtration. Soluble impurities remain in the cold solvent after recrystallization. The desired product should be as soluble as possible in hot solvent and as insoluble as possible in cold solvent. The selection of solvent is, therefore, critical to the successful recrystallization. Recrystallization is a purification procedure, which requires solubility of the impure solid in a heated solution and crystallization of the solid upon cooling. Clearly, this operation depends upon solute-solvent in traction involving a number of parameters including concentration, polarity of solute and solvent (like dissolves like), etc. Choice of a solvent or solvent pair for recrystallization experiments generally involves preliminary tests using a small sample and various solvent systems. To determine the proper solvent or solvent system, the following steps are commonly performed. I) The crude crystals should have low solubility in the chosen solvent at room temperature. II) The crude crystals should have high solubility in the chosen solvent when heated to boiling. III) The crude crystals should not react with the solvent IV) The solvent should boil at temperature below the solid melting point. V) The solvent should moderately be volatile so crystals dried readily. VI) The solvent should be non-toxic, non-flammable, and inexpensiveExperimental Procedures
Using a medicine dropper, place 0.15 to 0.20 g of aniline (about 10 drops) (d = 1.02 g/ml) in a large tared test tube and determine the weight to the nearest mg. Add 5 ml of distilled water to the test tube and then add 20 drops of acetic anhydride again using a medicine dropper (Fig.1). stir, the mixture using stirring rod for 5 minutes until solid forms. King Saud University Lap447CHEMEXPERIMENT 6
Preparation of Acetanilide
College Of Science
Chemistry Department
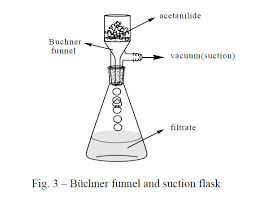
3 The product crystallized in the same test tube. Add 5 ml of water and heat the test tube in a hot water bath ( 400 mL beaker) (Fig.2) with occasional stirring until the entire solid dissolved. Set the test tube aside to cool for 3-5 minutes and then chill it in an ice bath. When crystallization is complete, collect the product by vacuum filtration using a small Büchner funnel (Fig.3). Allow the sample to dry completely. Weigh the dry product, calculate the percentage yield and determine its melting point. Collect to product in a paper and write your name and submit it to your instructor. The aqueous filtrate may be flushed down the drain. King Saud University Lap447CHEMEXPERIMENT 6
Preparation of Acetanilide
College Of Science
Chemistry Department
4Data and Results (Preparation and Purification of
Acetanilide)
Date:____________ Lab Report: _______
1. Sample name ________________________
2. Data on the impure sample
a. Mass of the aniline + test tube + beaker ________ g b. Mass of the aniline + test tube ________ g c. Mass of aniline ________ g d. Mole of aniline ________ mol e. Theoretical moles of Acetanilide ________ mol f. Theoretical mass of acetanilide ________ g (show calculation)3. Data for recrystallized acetanilide
a. Mass of recrystallized acetanilide + Weighing paper ________g b. Mass of recrystallized acetanilide ________g c. Calculation of percentage recovery (show calculation) ________% d. Melting point of recrystallized acetanilide ________ oC e. Structural formula of the sample recrystallizedquotesdbs_dbs4.pdfusesText_8[PDF] preparation of alcohol pdf
[PDF] preparation of benzoic acid by hydrolysis of ethyl benzoate
[PDF] preparation of buffer solution pdf
[PDF] preparation of esters lab answers grade 12
[PDF] preparation of lab reagents pdf
[PDF] preparation of laboratory solutions
[PDF] preparation of shampoo pdf
[PDF] preparation of sulphaguanidine from aniline
[PDF] preparation of trainers
[PDF] preparing for your acs examination in organic chemistry
[PDF] preposition combinations with adjectives and verbs
[PDF] preposition examples meaning
[PDF] preposition exercises upper intermediate pdf
[PDF] preposition exercises with answers pdf
